Chemistry:Copper benzoate
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
copper dibenzoate
| |
| Other names
cupric benzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H10CuO4 | |
| Molar mass | 305.7728 g/mol |
| Appearance | blue solid |
| Density | 1.197g/cm3 |
| Hazards | |
| Flash point | 111.4 °C (232.5 °F; 384.5 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[1] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[1] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[1] |
| Related compounds | |
Other cations
|
sodium benzoate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper benzoate is the chemical compounds with the formula Cu(C6H5CO2)2(H2O)x. These coordination complexes are derived from the cupric ion and the conjugate base of benzoic acid. Many derivatives are known with diverse ancillary ligands. This compound has found some use as a source of blue light in fireworks.
Preparation
In laboratory, copper benzoate can be made by combining aqueous solutions of potassium benzoate with copper sulfate. Hydrated copper benzoate precipitates as a pale blue solid:
- 4 C
6H
5CO
2K + 2 CuSO
4 · 5H2O → Cu
2(C
6H
5CO
2)
4(H
2O)
2 + 2 K
2SO
4 + 8 H
2O
The primary use of this compound is in production of blue flame in fireworks. Copper benzoate made from sodium benzoate for use in fireworks may result in strong yellow dilution of the flame unless the precipitate is carefully washed to remove sodium ion (which emits brightly yellow). Emission from potassium does not complicate the emission spectrum.[2]-->
Related compounds
Structure
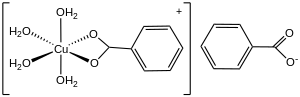
Copper(II) benzoates exists in at least two structural forms, depending on the degree of hydration. As in copper(II) acetate, one form of copper benzoate adopts a "Chinese lantern" structure,[3] wherein a pair of copper centers are linked by four bridging carboxylate ligands. Typically, one site on each copper center is occupied by water, which can be replaced by other ligands.[4] A hydrated form is also known, wherein each Cu(II) centre is bound to four water ligands and a bidentate O,O-benzoate.[5]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0150.html.
- ↑ "Wouter's Practical Pyrotechnics page". http://www.wfvisser.dds.nl/EN/cheminfo_EN.html#copperbenzoate.
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN:0-19-855370-6.
- ↑ T. Kawata, H. Uekusa, S. Ohba, T. Furukawa, T. Tokii, Y. Muto, M. Kato "Magneto-structural correlation in dimeric copper(II) benzoates" Acta Crystallogr. 1992, vol. B48, pp. 253-261. doi:10.1107/S0108768191012697
- ↑ Koizumi, Hideo; Osaki, Kenji; Watanabé, Tokunosuké (1963). "Crystal Structure of Cupric Benzoate Trihydrate Cu(C6H5COO)23H2O". Journal of the Physical Society of Japan 18 (1): 117–124. doi:10.1143/JPSJ.18.117. Bibcode: 1963JPSJ...18..117K.
 |


