Chemistry:Diruthenium tetraacetate chloride
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Ruthenium(II,III) acetate chloride
| |
| Other names
Tetrakis(mu-(acetato-O:O'))chlorodiruthenium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C8H12ClO8Ru2 | |
| Molar mass | 473.77 g·mol−1 |
| Appearance | red-brown solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
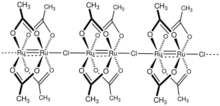
Diruthenium tetraacetate chloride is the coordination polymer with the formula {[Ru2(O2CCH3)4]Cl}n. A red brown solid, the compound is obtained by the reduction of ruthenium trichloride in acetic acid.[1] The compound has attracted much academic interest because it features a fractional metal-metal bond order of 2.5.[2]
The [Ru2(O2CCH3)4]+ core adopts the Chinese lantern structure, with four acetate ligands spanning the Ru2 center. The Ru-Ru distance is 228 pm.[3][4] The [Ru2(O2CCH3)4]+ cages are linked by bridging chloride ligands.
References
- ↑ Mitchell, Robert W.; Spencer, Alwyn; Wilkinson, Geoffrey (1973). "Carboxylato-Triphenylphosphine complexes of Ruthenium, Cationic Triphenylphosphine Complexes Derived from Them, and Their Behaviour as homogeneous hydrogenation Catalysts for Alkenes". Journal of the Chemical Society, Dalton Transactions (8): 846. doi:10.1039/DT9730000846.
- ↑ Aquino, Manuel A.S. (1998). "Diruthenium and Diosmium Tetracarboxylates: Synthesis, Physical Properties and Applications". Coordination Chemistry Reviews 170: 141–202. doi:10.1016/S0010-8545(97)00079-9.
- ↑ Cotton, F.Albert; Kim, Youngmee; Ren, Tong (1993). "Molecular Structure and Magnetic Properties of a Linear Chain Compound, Ru2(O2CCMePh2)4Cl". Polyhedron 12 (6): 607–611. doi:10.1016/S0277-5387(00)84975-X.
- ↑ Martin, Don S.; Newman, Robert A.; Vlasnik, Lynn M. (1980). "Crystal structure and polarized electronic spectra for diruthenium tetraacetate chloride". Inorganic Chemistry 19 (11): 3404–3407. doi:10.1021/ic50213a038. ISSN 0020-1669. https://pubs.acs.org/doi/pdf/10.1021/ic50213a038.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

