Chemistry:Rhodium(II) acetate
| |

| |
| Names | |
|---|---|
| IUPAC name
Rhodium(II) acetate
| |
| Other names
Dirhodium tetraacetate,
Tetrakis(acetato)dirhodium(II), Rhodium diacetate dimer, Tetrakis(μ-acetato)dirhodium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C8H12O8Rh2 | |
| Molar mass | 441.99 g/mol |
| Appearance | Emerald green powder |
| Density | 1.126 g/cm3 |
| Melting point | >100 °C |
| Boiling point | decomposes |
| soluble | |
| Solubility in other solvents | polar organic solvents |
| Structure | |
| monoclinic | |
| octahedral | |
| 0 D | |
| Hazards | |
| Safety data sheet | Coleparmer MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
| NFPA 704 (fire diamond) | |
| Flash point | low flammability |
| Related compounds | |
Related compounds
|
Copper(II) acetate Chromium(II) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (CH3CO−2). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alkenes. It is a widely studied example of a transition metal carboxylate complex.
Preparation
Rhodium(II) acetate is usually prepared by the heating of hydrated rhodium(III) chloride in acetic acid (CH3COOH):[2] Rhodium(II) acetate dimer undergoes ligand exchange, the replacement of the acetate group by other carboxylates and related groups.[3]
- Rh2(OAc)4 + 4 HO2CR → Rh2(O2CR)4 + 4 HOAc
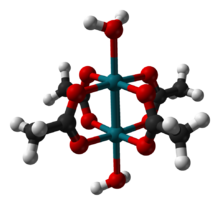
Structure and properties
The structure of rhodium(II) acetate features a pair of rhodium atoms, each with octahedral molecular geometry, defined by four acetate oxygen atoms, water, and a Rh–Rh bond of length 2.39 Å. The water adduct is exchangeable, and a variety of other Lewis bases bind to the axial positions.[4] Copper(II) acetate and chromium(II) acetate adopt similar structures.
Chemical properties
The application of dirhodium tetraacetate to organic synthesis was pioneered by Teyssie and co-workers.[5] An extensive range of reactions including insertion into bonds and the cyclopropanation of alkenes[6] and aromatic systems.[7] It selectively binds ribonucleosides (vs. deoxynucleosides) by binding selectively to ribonucleosides at their 2′ and 3′ –OH groups.[8] Rhodium(II) acetate dimer, compared to copper(II) acetate, is more reactive and useful in differentiating ribonucleosides and deoxynucleosides because it is soluble in aqueous solution like water whereas copper(II) acetate only dissolves in non-aqueous solution.
Selected catalytic reactions
Dirhodium tetraacetate is also used as catalyst for insertion into C–H and X–H bonds (X = N, S, O).
- Cyclopropanation

through the decomposition of diazocarbonyl compounds, the intra- and intermolecular cyclopropanation reactions occurs.
- Aromatic cycloaddition
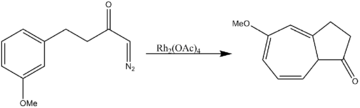
Rhodium acetate catalyzes both two-component cycloaddition as well as three-component 1,3-dipolar cycloadditions.
- C–H insertion
- Oxidation of alcohols

Allylic and benzylic alcohols were oxidized to the corresponding carbonyl compounds using tert-butyl hydroperoxide in stoichiometric amounts and Rh2(OAc)4 as catalyst in dichloromethane at ambient temperature.
- X–H insertion (X = N, S, O)
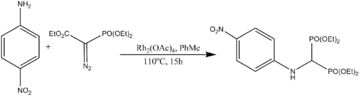
Rh(II) carbenoid reacts with amines, alcohols or thiols to yield the product of a formal intra- or intermolecular X–H bond (X = N, S, O) insertion via the formation of an ylide intermediate.
References
- ↑ "Dirhodium tetraacetate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/152122#section=Safety-and-Hazards.
- ↑ Rempel, G. A.; Legzdins, P.; Smith, H.; Wilkinson, G. (1972). "Tetrakis(acetato)dirhodium(II) and Similar Carboxylato Compounds". Inorganic Syntheses. 13. 90–91. doi:10.1002/9780470132449.ch16. ISBN 9780470132449.
- ↑ Doyle, M. P. (2000). "Asymmetric Addition and Insertion Reactions of Catalytically-Generated Metal Carbenes". in Ojima, Iwao. Catalytic Asymmetric Synthesis (2nd ed.). New York: Wiley. ISBN 978-0-471-29805-2.
- ↑ Cotton, F. A.; Deboer, B. G.; Laprade, M. D.; Pipal, J. R.; Ucko, D. A. (1971). "The crystal and molecular structures of dichromium tetraacetate dihydrate and dirhodium tetraacetate dihydrate". Acta Crystallogr B 27 (8): 1664. doi:10.1107/S0567740871004527.
- ↑ Paulissen, R.; Reimlinger, H.; Hayez, E.; Hubert, A. J.; Teyssié, P. (1973). "Transition metal catalysed reactions of diazocompounds. II: Insertion in the hydroxylic bond". Tetrahedron Lett. 14 (24): 2233. doi:10.1016/S0040-4039(01)87603-6. https://orbi.uliege.be/handle/2268/236113.
- ↑ Hubert, A. J.; Feron, A.; Warin, R.; Teyssie, P. (1976). "Synthesis of iminoaziridines from carbodiimides and diazoesters : A new example of transition metal salt catalysed reactions of carbenes". Tetrahedron Lett. 17 (16): 1317. doi:10.1016/S0040-4039(00)78050-6. https://orbi.uliege.be/handle/2268/236097.
- ↑ Anciaux, A. J.; Demonceau, A.; Hubert, A. J.; Noels, A. F.; Petiniot, N.; Teyssié, P. (1980). "Catalytic control of reactions of dipoles and carbenes; an easy and efficient synthesis of cycloheptatrienes from aromatic compounds by an extension of Buchner's reaction". J. Chem. Soc., Chem. Commun. (16): 765–766. doi:10.1039/C39800000765. https://orbi.uliege.be/handle/2268/237699.
- ↑ Berger, N. A.; Tarien, E.; Eichhorn, G. L. (1972). "Stereoselective Differentiation between Ribonucleosides and Deoxynucleosides by Reaction with the Copper(II) Acetate Dimer". Nature New Biology 239 (95): 237–40. doi:10.1038/newbio239237a0. PMID 4538853.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |



