Chemistry:Leinamycin
| |
| Names | |
|---|---|
| IUPAC name
(2R,4′R,6R,9E,11R,13E,15Z)-4′,11-Dihydroxy-2,4′,9-trimethyl-1′-oxospiro[19-thia-3,20-diazabicyclo[15.2.1]icosa-1(20),9,13,15,17-pentaene-6,5′-dithiolane]-3′,4,12-trione
| |
| Other names
Streptomyces antibiotic DC-107
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C22H26N2O6S3 | |
| Molar mass | 510.64 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
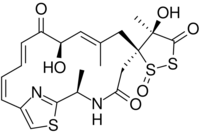
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria.[1] The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.[2][3]
Biosynthesis
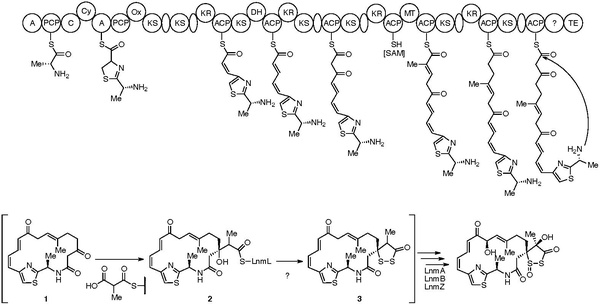
The seminal proposal for the biosynthesis of leinamycin was published in Chemistry & Biochemistry in 2004.[4] This biosynthesis consists of a discrete and modular NRPS, AT-less PKSs, and PKS modules. NRPS-PKS assembly line dictates the loading of D-Ala to initiate biosynthesis, followed by the loading of L-Cys to the peptidyl carrier protein (PCP). The dipeptide is then cyclized and oxidized to form the thiazonyl-S-PCP intermediate. The thiazonyl intermediate is then transferred to the PKS assembly line where the backbone is elongated by six units. The leinamycin hybrid peptide-polyketide carbon backbone intermediate is then cyclized by the thioesterase domain (TE) to yield intermediate 1. Methylmalonyl-CoA then condenses at the beta-keto group of 1, yielding 2. A series of tailoring enzymes converts 2 to 4, presumably through intermediate 3 to complete the biosynthesis of leinamycin.
References
- ↑ Kara, Mitsunobu; Asano, Kozo; Kawamoto, Isao; Takiouchi, Toshimitsu; Katsumata, Shigeo; Takahashi, KEI-Ichi; Nakano, Hirofumi (1989). "Leinamycin, a new antitumor antibiotic from Streptomyces; Producing organism, fermentation and isolation". The Journal of Antibiotics 42 (12): 1768–1774. doi:10.7164/antibiotics.42.1768. PMID 2621160.
- ↑ Hara, Mitsunobu; Saitoh, Yutaka; Nakano, Hirofumi (1990). "DNA strand scission by the novel antitumor antibiotic leinamycin". Biochemistry 29 (24): 5676–5681. doi:10.1021/bi00476a005. PMID 2383554.
- ↑ Asai, Akira; Hara, Mitsunobu; Kakita, Shingo; Kanda, Yutaka; Yoshida, Mayumi; Saito, Hiromitsu; Saitoh, Yutaka (1996). "Thiol-Mediated DNA Alkylation by the Novel Antitumor Antibiotic Leinamycin". Journal of the American Chemical Society 118 (28): 6802–6803. doi:10.1021/ja960892w.
- ↑ Tang, Gong-Li; Cheng, Yi-Qiang; Shen, Ben (2004). "Leinamycin Biosynthesis Revealing Unprecedented Architectural Complexity for a Hybrid Polyketide Synthase and Nonribosomal Peptide Synthetase". Chemistry & Biology 11 (1): 33–45. doi:10.1016/j.chembiol.2003.12.014. PMID 15112993.
 |