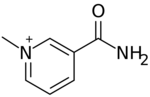
Chemistry:1-Methylnicotinamide
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Carbamoyl-1-methylpyridin-1-ium | |
| Other names
Trigonellamide; N1-Methylnicotinamide; NMN
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C7H9N2O+ | |
| Molar mass | 137.161 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Methylnicotinamide (trigonellamide) is a prototypic organic cation.[1] 1-Methylnicotinamide is the methylated amide of Nicotinamide (niacinamide, vitamin B3).
1-Methylnicotinamide is an endogenic substance that is produced in the liver when Nicotinamide is metabolized. It is a typical substance secreted in the kidney.
Occurrence
The highest natural concentration of 1-methylnicotinamide found so far is in the alga Undaria pinnatifida.[2] 1-Methylnicotinamide is also present in the Judas' ear fungus and in green tea.[2]
Extraction and production
1-Methylnicotinamide can be produced in the liver by nicotinamide N-methyltransferase. The reaction takes place during the metabolism of NAD (nicotinamide adenine dinucleotide).
NNMT (nicotinamide N-methyltransferase) is an enzyme that in humans is encoded by the NNMT gene.[3] NNMT catalyzes the methylation of nicotinamide and similar compounds using the methyl donor S-adenosyl methionine (SAM-e) to produce S-adenosyl-L-homocysteine (SAH) and 1-methylnicotinamide.[4] NNMT is highly expressed in the human liver.[4]
Use
For a long time, 1-methylnicotinamide was considered a biologically inactive metabolite of nicotinamide. However, various studies show antithrombotic,[5] anti-inflammatory,[6] gastroprotective[7] and vasoprotective[7] properties.
1-Methylnicotinamide is an endogenous activator of prostacyclin synthesis and can therefore regulate thrombolytic[check spelling] and inflammatory processes in the cardiovascular system.[8] It inhibits platelet-dependent thrombosis through a mechanism involving[9] cyclooxygenase-2 and prostacyclin and increases nitric oxide bioavailability in the endothelium.[7][4]
Animal experiments with diabetic rats have shown that 1-methylnicotinamide has a positive effect on degenerative changes in the brain and cognitive performance can be thus longer maintained.[10]
Experiments with the nematode Caenorhabditis elegans showed that the addition of 1-methylnicotinamide can extend their lifespan. This may possibly be attributed to increased free radical binding and the resulting reduced oxidative stress.[11]
1-Methylnicotinamide is used in cosmetic products such as hair- and skincare products and as a dietary supplement.[12]
References
- ↑ Sokol, P. P.; Holohan, P. D.; Ross, C. R. (1986). "Essential disulfide and sulfhydryl groups for organic cation transport in renal brush-border membranes". Journal of Biological Chemistry 261 (7): 3282–3287. doi:10.1016/S0021-9258(17)35779-4. PMID 2936734. https://www.jbc.org/article/S0021-9258(17)35779-4/pdf. Retrieved 5 March 2021.
- ↑ 2.0 2.1 Taguchi, H.; Sakaguchi, M.; Shimabayashi, Y. (1986). (in ja)ビタミン 60 (11): 537–546. doi:10.20632/vso.60.11_537.
- ↑ "Human nicotinamide N-methyltransferase gene: molecular cloning, structural characterization and chromosomal localization". Genomics 29 (3): 555–561. Mar 1996. doi:10.1006/geno.1995.9966. PMID 8575745.
- ↑ 4.0 4.1 4.2 Pissios P (2017). "Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme". Trends in Endocrinology and Metabolism 28 (5): 340–353. doi:10.1016/j.tem.2017.02.004. PMID 28291578.
- ↑ Gębicki, J.; Sysa-Jędrzejowska, A.; Adamus, J.; Woźniacka, A.; Rybak, M.; Zielonka, J. (2003). "1-Methylnicotinamide: A potent anti-inflammatory agent of vitamin origin". Polish Journal of Pharmacology 55 (1): 109–112. PMID 12856834. http://www.if-pan.krakow.pl/pjp/pdf/2003/1_109.pdf.
- ↑ Bryniarski, K.; Biedron, R.; Jakubowski, A.; Chlopicki, S.; Marcinkiewicz, J. (2008). "Anti-inflammatory effect of 1-methylnicotinamide in contact hypersensitivity to oxazolone in mice; involvement of prostacyclin". European Journal of Pharmacology 578 (2–3): 332–338. doi:10.1016/j.ejphar.2007.09.011. PMID 17935712.
- ↑ 7.0 7.1 7.2 Domagala, T. B.; Szeffler, A.; Dobrucki, L. W.; Dropinski, J.; Polanski, S.; Leszczynska-Wiloch, M.; Kotula-Horowitz, K.; Wojciechowski, J. et al. (2012). "Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N1-methylnicotinamide in human blood vessels". Hypertension 59 (4): 825–832. doi:10.1161/HYPERTENSIONAHA.111.183210. PMID 22353616.
- ↑ Bartuś, M.; Łomnicka, M.; Kostogrys, R. B.; Kaźmierczak, P.; Watała, C.; Słominska, E. M.; Smoleński, R. T.; Pisulewski, P. M. et al. (2008). "1-Methylnicotinamide (MNA) prevents endothelial dysfunction in hypertriglyceridemic and diabetic rats". Pharmacological Reports 60 (1): 127–138. PMID 18276994. http://www.if-pan.krakow.pl/pjp/pdf/2008/1_127.pdf.
- ↑ Chlopicki, S.; Swies, J.; Mogielnicki, A.; Buczko, W.; Bartus, M.; Lomnicka, M.; Adamus, J.; Gebicki, J. (2007). "1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway". British Journal of Pharmacology 152 (2): 230–239. doi:10.1038/sj.bjp.0707383. PMID 17641676.
- ↑ Kuchmerovska, T.; Shymanskyy, I.; Chlopicki, S.; Klimenko, A. (2010). "1-Methylnicotinamide (MNA) in prevention of diabetes-associated brain disorders". Neurochemistry International 56 (2): 221–228. doi:10.1016/j.neuint.2009.10.004. PMID 19837120.
- ↑ Schmeisser, K.; Mansfeld, J.; Kuhlow, D.; Weimer, S. et al. (2013). "Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide". Nature Chemical Biology 9 (11): 693–700. doi:10.1038/nchembio.1352. PMID 24077178.
- ↑ "Menavitin Produkte, die das Molekül 1-MNA beinhalten: das Novel Food mit natürlicher Intelligenz" (in de). 21 November 2019. https://www.startupvalley.news/de/menativin-1-mna-novel-food/.
 |

