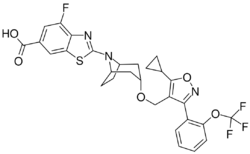
Chemistry:Tropifexor
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C29H25F4N3O5S |
| Molar mass | 603.59 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tropifexor is an investigational drug that acts as an agonist of the farnesoid X receptor (FXR). It was discovered by researchers from Novartis and Genomics Institute of the Novartis Research Foundation. Its synthesis and pharmacological properties were published in 2017.[1] It was developed for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). In combination with cenicriviroc, a CCR2 and CCR5 receptor inhibitor, it is undergoing a phase II clinical trial for NASH and liver fibrosis.[2]
Rats treated orally with tropifexor (0.03 to 1 mg/kg) showed an upregulation of the FXR target genes, BSEP and SHP, and a down-regulation of CYP8B1. Its EC50 for FXR is between 0.2 and 0.26 nM depending on the biochemical assay.
The patent that covers tropifexor and related compounds was published in 2010.[3]
References
- ↑ "Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH)". Journal of Medicinal Chemistry 60 (24): 9960–9973. December 2017. doi:10.1021/acs.jmedchem.7b00907. PMID 29148806.
- ↑ Clinical trial number NCT03517540 for "Safety, Tolerability, and Efficacy of a Combination Treatment of Tropifexor (LJN452) and Cenicriviroc (CVC) in Adult Patients With Nonalcoholic Steatohepatitis (NASH) and Liver Fibrosis. (TANDEM)" at ClinicalTrials.gov
- ↑ Alper PB, Chianelli D, Mutnick D, Vincent P, Tully DC, "Compositions and methods for modulating fxr", WO patent Application Filing 2012087519, published 2012-06-28, assigned to Genomics Institute of the Novartis Research Foundation. Retrieved 17 May 2019.
 |

