Chemistry:Cenicriviroc
| |
| Names | |
|---|---|
| Preferred IUPAC name
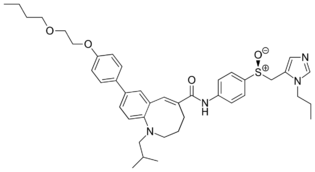
(5E)-8-[4-(2-Butoxyethoxy)phenyl]-1-(2-methylpropyl)-N-{4-[(S)-(1-propyl-1H-imidazol-5-yl)methanesulfinyl]phenyl}-1,2,3,4-tetrahydro-1-benzazocine-5-carboxamide | |
| Other names
TAK-652; TBR-652
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C41H52N4O4S | |
| Molar mass | 696.95 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cenicriviroc (INN,[1] code names TAK-652, TBR-652, commonly abbreviated as CVC) is an experimental drug candidate for the treatment of HIV infection[2] and in combination with Tropifexor for non-alcoholic steatohepatitis.[3] It is being developed by Takeda and Tobira Therapeutics.[citation needed]
Cenicriviroc is an inhibitor of CCR2 and CCR5 receptors,[4] allowing it to function as an entry inhibitor which prevents the virus from entering into a human cell. Inhibition of CCR2 may have an anti-inflammatory effect.[citation needed]
A double-blind, randomized, placebo-controlled clinical study to assess the antiviral activity, safety, and tolerability of cenicriviroc was conducted in 2010. HIV-infected patients taking cenicriviroc had significant reductions in viral load, with the effect persisting up to two weeks after discontinuation of treatment.[5] Additional Phase II clinical trials are underway.[6][when?]
Cenicriviroc is also in two separate clinical trials for COVID-19: the ACTIV-I trial run by the National Center for Advancing Translational Sciences, where it is compared with a number of other immunomodulatory agents,[7] and the Charité Trial of Cenicriviroc at the Charité Hospital in Berlin.[8] (As of July 2021), both trials are recruiting participants, and are expected to complete in September 2021.[citation needed]
Phase IIb data presented at the 20th Conference on Retroviruses and Opportunistic Infections (CROI) in March 2013 showed similar viral suppression rates of 76% for patients taking 100 mg cenicriviroc, 73% with 200 mg cenicriviroc, and 71% with efavirenz. Non-response rates were higher with cenicriviroc, however, largely due to greater drop-out of patients. A new tablet formulation with lower pill burden may improve adherence. Looking at immune and inflammatory biomarkers, levels of MCP-1 increased and soluble CD14 decreased in the cenicriviroc arms.[9]
See also
- Discovery and development of CCR5-receptor antagonists
- Maraviroc
- Vicriviroc
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 65". World Health Organization. 2011. pp. 53–4. https://www.who.int/medicines/publications/druginformation/innlists/RL65.pdf.
- ↑ Klibanov, OM; Williams, SH; Iler, CA (August 2010). "Cenicriviroc, an Orally Active CCR5 Antagonist for the Potential Treatment of HIV Infection". Current Opinion in Investigational Drugs 11 (8): 940–50. PMID 20721836.
- ↑ "A Randomized, Double-blind, Multicenter Study to Assess the Safety, Tolerability, and Efficacy of a Combination Treatment of Tropifexor (LJN452) and Cenicriviroc (CVC) in Adult Patients with Nonalcoholic Steatohepatitis (NASH) and Liver Fibrosis". 21 January 2022. https://clinicaltrials.gov/ct2/show/NCT03517540.
- ↑ Baba, M; Takashima, K; Miyake, H; Kanzaki, N; Teshima, K; Wang, X; Shiraishi, M; Iizawa, Y (26 October 2005). "TAK-652 Inhibits CCR5-Mediated Human Immunodeficiency Virus Type 1 Infection In Vitro and Has Favorable Pharmacokinetics in Humans". Antimicrobial Agents and Chemotherapy 49 (11): 4584–91. doi:10.1128/AAC.49.11.4584-4591.2005. PMID 16251299.
- ↑ Reviriego, C (July 2011). "Chemokine CCR2/CCR5 Receptor Antagonist Anti-HIV Agent". Drugs of the Future 36 (7): 511–7. doi:10.1358/dof.2011.036.07.1622066.
- ↑ "Tobira Therapeutics Initiates Phase 2b Trial of Cenicriviroc". The Body. July 5, 2011. http://www.thebody.com/content/62837/tobira-therapeutics-initiates-phase-2b-trial-of-ce.html.
- ↑ Benjamin, Daniel (2021-06-29). Randomized Master Protocol for Immune Modulators for Treating COVID-19. Daniel Benjamin, National Center for Advancing Translational Science (NCATS), Biomedical Advanced Research and Development Authority. https://clinicaltrials.gov/ct2/show/NCT04593940.
- ↑ Tacke, Frank (2020-08-25). Charité Trial of Cenicriviroc (CVC) Treatment for COVID-19 Patients. Charite University, Berlin, Germany, Allergan. https://clinicaltrials.gov/ct2/show/NCT04500418.
- ↑ CROI 2013: CCR5/CCR2 Inhibitor Cenicriviroc Has Both Anti-HIV and Anti-inflammatory Effects. Highleyman, Liz. HIVandHepatitis.com. 7 March 2013.
 |

