Chemistry:Fosigotifator
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | ABBV-CLS-7262; Fosigotifator sodium tromethamine |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
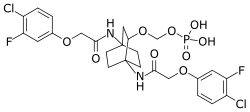
| Formula | C25H27Cl2F2N2O9P |
| Molar mass | 639.37 g·mol−1 |
Fosigotifator is an experimental small-molecule developed by AbbVie, which is running clinical trials to determine if the drug can treat amyotrophic lateral sclerosis (ALS).[1][2][3] A formulation of fosigotifator, as its monosodium phosphate salt mixed with tromethamine, is known as ABBV-CLS-7262. Fosigotifator has been patented by AbbVie and Calico Life Sciences as a prodrug for modulation of the integrated stress response pathway.[4]
References
- ↑ Arnold, F. J.; Nguyen, A. D.; Bedlack, R. S.; Bennett, C. L.; La Spada, A. R. (1 August 2023). "Intercellular transmission of pathogenic proteins in ALS: Exploring the pathogenic wave". Neurobiology of Disease 184: 106218. doi:10.1016/j.nbd.2023.106218. ISSN 0969-9961. PMID 37394036.
- ↑ Martinez-Gonzalez, Loreto; Martinez, Ana (1 February 2023). "Emerging clinical investigational drugs for the treatment of amyotrophic lateral sclerosis" (in en). Expert Opinion on Investigational Drugs 32 (2): 141–160. doi:10.1080/13543784.2023.2178416. ISSN 1354-3784. PMID 36762798.
- ↑ Cho, William; Jeong, Anna; Malik, Paul; Boiser, Joey; Huang, Xiu; Rosebraugh, Matthew (25 April 2023). "A Phase 1 First-in-human Study to Investigate the Safety, Tolerability and Food Effect of ABBV-CLS-7262 (P6-4.002)" (in en). Neurology 100 (17 Supplement 2): 4188. doi:10.1212/WNL.0000000000203810. ISSN 0028-3878.
- ↑ "Prodrug Modulators of the Integrated Stress Pathway" WO patent 2020077217, published 2020-04-16
 |

