Chemistry:Oxetane
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxetane[1] | |||
| Systematic IUPAC name
1,3-Epoxypropane Oxacyclobutane | |||
| Other names
1,3-Propylene oxide
Trimethylene oxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102382 | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| 239520 | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1280 | ||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.08 g/mol | ||
| Density | 0.8930 g/cm3 | ||
| Melting point | −97 °C (−143 °F; 176 K) | ||
| Boiling point | 49 to 50 °C (120 to 122 °F; 322 to 323 K) | ||
Refractive index (nD)
|
1.3895 at 25°C | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H225, H302, H312, H332 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+312, P302+352, P303+361+353, P304+312, P304+340, P312, P322, P330, P363, P370+378, P403+235, P501 | |||
| Flash point | −28.3 °C; −19.0 °F; 244.8 K (NTP, 1992) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
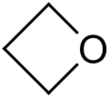
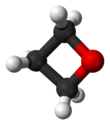
Oxetane, or 1,3-propylene oxide, is a heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom.
The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring.
Production
A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C:[2]
Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products including water, potassium chloride, and potassium acetate.
Another possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization as well as through decarboxylation of a six-membered cyclic carbonate.[clarification needed]
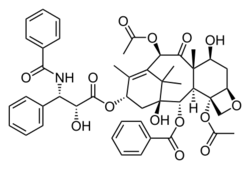
Taxol
Paclitaxel (Taxol) is an example of a natural product containing an oxetane ring. Taxol has become a major point of interest among researchers due to its unusual structure and success in the involvement of cancer treatment.[3] The attached oxetane ring is an important feature that is used for the binding of microtubules in structure activity; however little is known about how the reaction is catalyzed in nature, which creates a challenge for scientists trying to synthesize the product.[3]
Derivatives
More than a hundred different oxetanes have been synthesized.[4] Functional groups can be added into any desired position in the oxetane ring, including fully fluorinated (perfluorinated) and fully deuterated analogues. Major examples are listed in table below.
| Name | Structure | Abbreviation | Boiling point, Bp [°C] |
|---|---|---|---|
| 3,3-Bis(chloromethyl)oxetane | 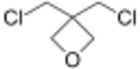
|
BCMO | 198[5] |
| 3,3-Bis(azidomethyl)oxetane | 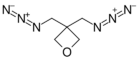
|
BAMO | 165[6] |
| 2-Methyloxetane | 
|
2MOX | 60[4] |
| 3-Methyloxetane | 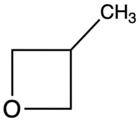
|
3MOX | 67[4] |
| 3-Azidooxetane | 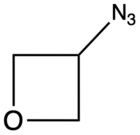
|
AZOX | 122[7] |
| 3-Nitrooxetane | 
|
NIOX | 195[8] |
| 3,3-Dimethyloxetane | 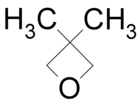
|
DMOX | 80[4] |
| 3,3-Dinitrooxetane | 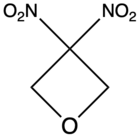
|
DNOX | – |
See also
- β-Propiolactone or 2-oxetanone.
- 3-Oxetanone
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ C. R. Noller (1955). "Trimethylene Oxide". Organic Syntheses 29: 92. http://www.orgsyn.org/demo.aspx?prep=CV3P0835.; Collective Volume, 3, pp. 835
- ↑ 3.0 3.1 Willenbring, Dan; Tantillo, Dean J. (April 2008). "Mechanistic possibilities for oxetane formation in the biosynthesis of Taxol's D ring". Russian Journal of General Chemistry 78 (4): 723–731. doi:10.1134/S1070363208040336.
- ↑ 4.0 4.1 4.2 4.3 Goethals, Lucien; Sioen, Philip (2000). "Dialogos. Lucien Goethals in gesprek met Philip Sioen". Revue belge de Musicologie / Belgisch Tijdschrift voor Muziekwetenschap 54: 49–71. doi:10.2307/3686877. ISSN 0771-6788. http://dx.doi.org/10.2307/3686877.
- ↑ "78-71-7 CAS MSDS (3,3-BIS(CHLOROMETHYL)OXETANE) Melting Point Boiling Point Density CAS Chemical Properties". https://www.chemicalbook.com/ChemicalProductProperty_US_CB5718355.aspx.
- ↑ Akhtar, Tauseef; Berger, Ronald; Marine, Joseph E; Daimee, Usama A; Calkins, Hugh; Spragg, David (2020-08-13). "Cryoballoon Ablation of Atrial Fibrillation in Octogenarians". Arrhythmia & Electrophysiology Review 9 (2): 104–107. doi:10.15420/aer.2020.18. ISSN 2050-3377. PMID 32983532.
- ↑ Baum, Kurt; Berkowitz, Phillip T.; Grakauskas, Vytautas; Archibald, Thomas G. (September 1983). "Synthesis of electron-deficient oxetanes. 3-Azidooxetane, 3-nitrooxetane, and 3,3-dinitrooxetane". The Journal of Organic Chemistry 48 (18): 2953–2956. doi:10.1021/jo00166a003. ISSN 0022-3263. http://dx.doi.org/10.1021/jo00166a003.
- ↑ "3-Nitrooxetane | C3H5NO3 | ChemSpider". https://www.chemspider.com/Chemical-Structure.14636403.html.
 |