Chemistry:Thymolphthalein
From HandWiki
| |
| Names | |
|---|---|
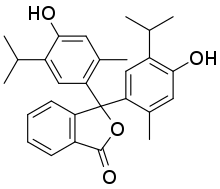
| Preferred IUPAC name
3,3-Bis[4-hydroxy-2-methyl-5-(propan-2-yl)phenyl]-2-benzofuran-1(3H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H30O4 | |
| Molar mass | 430.544 g·mol−1 |
| Appearance | White powder |
| Melting point | 248 to 252 °C (478 to 486 °F; 521 to 525 K) (decomposes) |
| Hazards[1] | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H341, H350, H361 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P280, P281, P303+361+353, P308+313, P370+378, P403+235, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thymolphthalein is a phthalein dye used as an acid–base (pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is 38,000 M−1 cm−1 at 595 nm.[2]
| Thymolphthalein (pH indicator) | ||
| below pH 9.3 | above pH 10.5 | |
| 9.3 | ⇌ | 10.5 |
Thymolphthalein is also known to have use as a laxative[3] and for disappearing ink.[4]
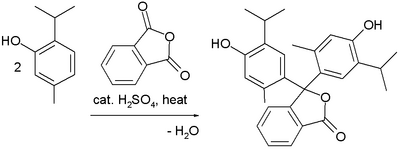
Preparation
Thymolphthalein can be synthesized from thymol and phthalic anhydride.
See also
References
- ↑ "Thymolphthalein" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/31316#section=Safety-and-Hazards.
- ↑ Hahn HH; Cheuk SF; Elfenbein S; Wood WB (April 1970). "Studies on the Pathogenesis of Fever: Xix. Localization of Pyrogen in Granulocytes". The Journal of Experimental Medicine 131 (4): 701–9. doi:10.1084/jem.131.4.701. PMID 5430784.
- ↑ Hubacher, MH; Doernberg, S; Horner, A (1953). "Laxatives: chemical structure and potency of phthaleins and hydroxyanthraquinones". Journal of the American Pharmaceutical Association 42 (1): 23–30. doi:10.1002/jps.3030420108. PMID 13034620.
- ↑ Katz, David A. (1982). "Disappearing Ink". https://www.chymist.com/Disappearing%20Ink.pdf.
 |