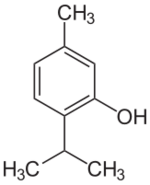
Chemistry:Thymol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Methyl-2-(propan-2-yl)phenol[1] | |
| Systematic IUPAC name
5-Methyl-2-(propan-2-yl)benzenol | |
| Other names
2-Isopropyl-5-methylphenol, isopropyl-m-cresol, 1-methyl-3-hydroxy-4-isopropylbenzene, 3-methyl-6-isopropylphenol, 5-methyl-2-(1-methylethyl)phenol, 5-methyl-2-isopropyl-1-phenol, 5-methyl-2-isopropylphenol, 6-isopropyl-3-methylphenol, 6-isopropyl-m-cresol, Apiguard, NSC 11215, NSC 47821, NSC 49142, thyme camphor, m-thymol, and p-cymen-3-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.221 g·mol−1 |
| Density | 0.96 g/cm3 |
| Melting point | 49 to 51 °C (120 to 124 °F; 322 to 324 K) |
| Boiling point | 232 °C (450 °F; 505 K) |
| 0.9 g/L (20 °C)[2] | |
Refractive index (nD)
|
1.5208[3] |
| Pharmacology | |
| 1=ATCvet code} | QP53AX22 (WHO) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H302, H314, H411 | |
| P260, P264, P270, P273, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thymol (also known as 2-isopropyl-5-methylphenol, IPMP), C
10H
14O, is a natural monoterpenoid phenol derivative of p-Cymene, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris (common thyme), ajwain,[4] and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris. Thymol is only slightly soluble in water at neutral pH, but it is extremely soluble in alcohols and other organic solvents. It is also soluble in strongly alkaline aqueous solutions due to deprotonation of the phenol. Its dissociation constant (pKa) is 10.59±0.10.[5] Thymol absorbs maximum UV radiation at 274 nm.[6]
Chemical synthesis
Thymol is produced by the alkylation of m-cresol and propene:[7][8]
- CH
3C
6H
4OH + CH
2CHCH
3 → ((CH
3)
2CH)CH
3C
6H
3OH
History
Ancient Egyptians used thyme for embalming.[9] The Ancient Greece used it in their baths and burned it as incense in their temples, believing it was a source of courage. The spread of thyme throughout Europe was thought to be due to the Romans, as they used it to purify their rooms and to "give an aromatic flavour to cheese and liqueurs".[10] In the European Middle Ages, the herb was placed beneath pillows to aid sleep and ward off nightmares.[11] In this period, women also often gave knights and warriors gifts that included thyme leaves, because it was believed to bring courage to the bearer. Thyme was also used as incense and placed on coffins during funerals, because it was supposed to ensure passage into the next life.[12]
The bee balms Monarda fistulosa and Monarda didyma, North American wildflowers, are natural sources of thymol. The Blackfoot Native Americans recognized these plants' strong antiseptic action and used poultices of the plants for skin infections and minor wounds. A tisane made from them was also used to treat mouth and throat infections caused by dental caries and gingivitis.[13]
Thymol was first isolated by German chemist Caspar Neumann in 1719.[14] In 1853, French chemist Alexandre Lallemand named thymol and determined its empirical formula.[15] Thymol was first synthesized by Swedish chemist Oskar Widman in 1882.[16]
Extraction
The conventional method of extracting is hydro-distillation (HD), but can also be extracted with solvent-free microwave extraction (SFME). In 30 minutes, SFME yields similar amounts of thymol with more oxygenated compounds than 4.5 hours of hydro-distillation at atmospheric pressures without the need for solvent.[17]
Uses

Thymol during the 1910s was the treatment of choice for hookworm infection in the United States.[18][19] People of the Middle East continue to use za'atar, a delicacy made with large amounts of thyme, to reduce and eliminate internal parasites.[20] It is also used as a preservative in halothane, an anaesthetic, and as an antiseptic in mouthwash. When used to reduce plaque and gingivitis, thymol has been found to be more effective when used in combination with chlorhexidine than when used purely by itself.[21] Thymol is also the active antiseptic ingredient in some toothpastes, such as Johnson & Johnson's Euthymol. Thymol has been used to successfully control varroa mites and prevent fermentation and the growth of mold in bee colonies.[22] Thymol is also used as a rapidly degrading, non-persisting pesticide.[23] Thymol can also be used as a medical disinfectant and general purpose disinfectant.[24] Thymol is also used in the production of menthol through the hydrogenation of the aromatic ring.[25]
List of plants that contain thymol
- Illicium verum
- Euphrasia rostkoviana[26]
- Lagoecia cuminoides[27]
- Monarda didyma[28]
- Monarda fistulosa[29]
- Mosla chinensis, Xiang Ru (香薷)
- Ocimum gratissimum L.[30]
- Origanum compactum[31]
- Origanum dictamnus[32]
- Origanum onites[33][34]
- Origanum vulgare[35][36]
- Satureja thymbra
- Thymus glandulosus[31]
- Thymus hyemalis[37]
- Thymus serpyllum
- Thymus praecox
- Thymus vulgaris[37][38]
- Thymus zygis[39]
- Trachyspermum ammi
Toxicology and environmental impacts
In 2009, the U.S. Environmental Protection Agency (EPA) reviewed the research literature on the toxicology and environmental impact of thymol and concluded that "thymol has minimal potential toxicity and poses minimal risk".[40]
Environmental breakdown and use as a pesticide
Studies have shown that hydrocarbon monoterpenes and thymol in particular degrade rapidly (DT50 16 days in water, 5 days in soil[23]) in the environment and are, thus, low risks because of rapid dissipation and low bound residues,[23] supporting the use of thymol as a pesticide agent that offers a safe alternative to other more persistent chemical pesticides that can be dispersed in runoff and produce subsequent contamination. Though, there has been recent research into sustained released systems for botanically derived pesticides, such as using natural polysaccharides which would be biodegradable and biocompatible.[41]
Compendial status
See also
- Thymoquinone
- Nigella sativa
- Bromothymol
Notes and references
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ "Thymol". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/thymol#section=Solubility.
- ↑ Mndzhoyan, A. L. (1940). "Thymol from Thymus kotschyanus.". Sbornik Trudov Armyanskogo Filial. Akad. Nauk. 1940: 25–28.
- ↑ O'Connell, John (27 August 2019). The book of spice : from anise to zedoary. New York: Pegasus. ISBN 978-1681774459. OCLC 959875923.
- ↑ CAS Registry: Data obtained from SciFinder[full citation needed]
- ↑ Norwitz, G.; Nataro, N.; Keliher, P. N. (1986). "Study of the Steam Distillation of Phenolic Compounds Using Ultraviolent Spectrometry". Anal. Chem. 58 (639–640): 641. doi:10.1021/ac00294a034.
- ↑ Stroh, R.; Sydel, R.; Hahn, W. (1963). Foerst, Wilhelm. ed. Newer Methods of Preparative Organic Chemistry, Volume 2 (1st ed.). New York: Academic Press. p. 344. ISBN 9780323150422. https://books.google.com/books?id=LG2J6i1sUAMC&pg=PA344.
- ↑ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ "A Brief History of Thyme - Hungry History". http://www.history.com/news/hungry-history/a-brief-history-of-thyme.
- ↑ Grieve, Mrs. Maud. "Thyme. A Modern Herbal". botanical.com. http://botanical.com/botanical/mgmh/t/thygar16.html.
- ↑ Huxley, A., ed. (1992). New RHS Dictionary of Gardening. Macmillan.
- ↑ "Thyme (thymus)". englishplants.co.uk. The English Cottage Garden Nursery. http://www.englishplants.co.uk/thyme.html.
- ↑ Tilford, Gregory L. (1997). Edible and Medicinal Plants of the West. Missoula, MT: Mountain Press Publishing. ISBN 978-0-87842-359-0.
- ↑ Neuman, Carolo (1724). "De Camphora". Philosophical Transactions of the Royal Society of London 33 (389): 321–332. doi:10.1098/rstl.1724.0061. http://rstl.royalsocietypublishing.org/content/33/381-391/321.full.pdf+html. On page 324, Neumann mentions that in 1719 he distilled some essential oils from various herbs. On page 326, he mentions that during these experiments, he obtained a crystalline substance from thyme oil, which he called "Camphora Thymi" (camphor of thyme). (Neumann gave the name "camphor" not only to the specific substance that today is called camphor but to any crystalline substance that precipitated from a volatile, fragrant oil from some plant.)
- ↑ Lallemand, A. (1853). "Sur la composition de l'huile essentielle de thym" (in fr). Comptes Rendus 37: 498–500. http://gallica.bnf.fr/ark:/12148/bpt6k29948/f502.image.langEN.
- ↑ Widmann, Oskar (1882). "Ueber eine Synthese von Thymol aus Cuminol" (in de). Berichte der Deutschen Chemischen Gesellschaft zu Berlin 15: 166–172. doi:10.1002/cber.18820150139. http://gallica.bnf.fr/ark:/12148/bpt6k90694n/f169.image.langEN.
- ↑ Lucchesi, Marie E; Chemat, Farid; Smadja, Jacqueline (2004-07-23). "Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation". Journal of Chromatography A 1043 (2): 323–327. doi:10.1016/j.chroma.2004.05.083. ISSN 0021-9673. https://www.sciencedirect.com/science/article/pii/S0021967304008672.
- ↑ Ferrell, John Atkinson (1914). The Rural School and Hookworm Disease. US Bureau of Education Bulletin. No. 20, Whole No. 593. Washington, DC: U.S. Government Printing Office. https://books.google.com/books?id=omYAAAAAYAAJ.
- ↑ Milton, Joseph Rosenau (1913). Preventive Medicine and Hygiene. D. Appleton. p. 119. https://books.google.com/books?id=mVfQAAAAMAAJ&pg=PA119.
- ↑ Inskeep, Steve; Godoy, Maria (2013-06-11). "Za'atar: A Spice Mix With Biblical Roots And Brain Food Reputation" (in en). NPR. https://www.npr.org/sections/thesalt/2013/06/11/190672515/zaatar-a-spice-mix-with-biblical-roots-and-brain-food-reputation.
- ↑ Filoche, S. K.; Soma, K.; Sissons, C. H. (2005). "Antimicrobial effects of essential oils in combination with chlorhexidine digluconate". Oral Microbiol. Immunol. 20 (4): 221–225. doi:10.1111/j.1399-302X.2005.00216.x. PMID 15943766.
- ↑ Ward, Mark (2006-03-08). "Almond farmers seek healthy bees". BBC. http://news.bbc.co.uk/2/hi/science/nature/4780034.stm.
- ↑ 23.0 23.1 23.2 Hu, D.; Coats, J. (2008). "Evaluation of the environmental fate of thymol and phenethyl propionate in the laboratory". Pest Manag. Sci. 64 (7): 775–779. doi:10.1002/ps.1555. PMID 18381775.
- ↑ "Thymol". US Environmental Protection Agency. September 1993. http://archive.epa.gov/pesticides/reregistration/web/pdf/3143fact.pdf.
- ↑ "Menthol | Definition, Structure, & Uses | Britannica" (in en). 2023-10-06. https://www.britannica.com/science/menthol.
- ↑ Novy, P.; Davidova, H.; Serrano Rojero, C. S.; Rondevaldova, J.; Pulkrabek, J.; Kokoska, L. (2015). "Composition and Antimicrobial Activity of Euphrasia rostkoviana Hayne Essential Oil". Evid Based Complement Alternat Med 2015: 1–5. doi:10.1155/2015/734101. PMID 26000025.
- ↑ Baser, K. H.C.; Tümen, G. (1994). "Composition of the Essential Oil of Lagoecia cuminoides L. from Turkey". Journal of Essential Oil Research 6 (5): 545–546. doi:10.1080/10412905.1994.9698448.
- ↑ Donata Ricci; Francesco Epifano; Daniele Fraternale (February 2017). Olga Tzakou. ed. "The Essential Oil of Monarda didyma L. (Lamiaceae) Exerts Phytotoxic Activity In Vitro against Various Weed Seeds". Molecules (Basel, Switzerland) (Molecules) 22 (2): 222. doi:10.3390/molecules22020222. PMID 28157176.
- ↑ Zamureenko, V. A.; Klyuev, N. A.; Bocharov, B. V.; Kabanov, V. S.; Zakharov, A. M. (1989). "An investigation of the component composition of the essential oil of Monarda fistulosa". Chemistry of Natural Compounds 25 (5): 549–551. doi:10.1007/BF00598073. ISSN 1573-8388.
- ↑ Escobar, Angélica; Pérez, Miriam; Romanelli, Gustavo; Blustein, Guillermo (2020-12-01). "Thymol bioactivity: A review focusing on practical applications". Arabian Journal of Chemistry 13 (12): 9243–9269. doi:10.1016/j.arabjc.2020.11.009. ISSN 1878-5352. https://www.sciencedirect.com/science/article/pii/S1878535220304561.
- ↑ 31.0 31.1 Bouchra, Chebli; Achouri, Mohamed; Idrissi Hassani, L. M.; Hmamouchi, Mohamed (2003). "Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr". Journal of Ethnopharmacology 89 (1): 165–169. doi:10.1016/S0378-8741(03)00275-7. PMID 14522450.
- ↑ Liolios, C. C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. (2009). "Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity". Food Chemistry 112 (1): 77–83. doi:10.1016/j.foodchem.2008.05.060.
- ↑ Ozkan, Gulcan; Baydar, H.; Erbas, S. (2009). "The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.)". Journal of the Science of Food and Agriculture 90 (2): 205–209. doi:10.1002/jsfa.3788. PMID 20355032.
- ↑ Lagouri, Vasiliki; Blekas, George; Tsimidou, Maria; Kokkini, Stella; Boskou, Dimitrios (1993). "Composition and antioxidant activity of essential oils from Oregano plants grown wild in Greece". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A 197 (1): 1431–4630. doi:10.1007/BF01202694.
- ↑ Kanias, G. D.; Souleles, C.; Loukis, A.; Philotheou-Panou, E. (1998). "Trace elements and essential oil composition in chemotypes of the aromatic plant Origanum vulgare". Journal of Radioanalytical and Nuclear Chemistry 227 (1–2): 23–31. doi:10.1007/BF02386426.
- ↑ Figiel, Adam; Szumny, Antoni; Gutiérrez Ortíz, Antonio; Carbonell Barrachina, Ángel A. (2010). "Composition of oregano essential oil (Origanum vulgare) as affected by drying method". Journal of Food Engineering 98 (2): 240–247. doi:10.1016/j.jfoodeng.2010.01.002.
- ↑ 37.0 37.1 Goodner, K.L.; Mahattanatawee, K.; Plotto, A.; Sotomayor, J.; Jordán, M. (2006). "Aromatic profiles of Thymus hyemalis and Spanish T. vulgaris essential oils by GC–MS/GC–O". Industrial Crops and Products 24 (3): 264–268. doi:10.1016/j.indcrop.2006.06.006.
- ↑ Lee, Seung-Joo; Umano, Katumi; Shibamoto, Takayuki; Lee, Kwang-Geun (2005). "Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties". Food Chemistry 91 (1): 131–137. doi:10.1016/j.foodchem.2004.05.056.
- ↑ Moldão Martins, M.; Palavra, A.; Beirão da Costa, M. L.; Bernardo Gil, M. G. (2000). "Supercritical CO2 extraction of Thymus zygis L. subsp. sylvestris aroma". The Journal of Supercritical Fluids 18 (1): 25–34. doi:10.1016/S0896-8446(00)00047-4.
- ↑ 74 FR 12613
- ↑ Campos, Estefânia V. R.; Proença, Patrícia L. F.; Oliveira, Jhones L.; Bakshi, Mansi; Abhilash, P. C.; Fraceto, Leonardo F. (2019-10-01). "Use of botanical insecticides for sustainable agriculture: Future perspectives". Ecological Indicators 105: 483–495. doi:10.1016/j.ecolind.2018.04.038. ISSN 1470-160X. https://www.sciencedirect.com/science/article/pii/S1470160X18302917.
- ↑ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf.
- ↑ "Japanese Pharmacopoeia". http://jpdb.nihs.go.jp/jp15e/JP15.pdf.
External links
 |
