Chemistry:Sodium hexachloroosmate
From HandWiki
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl6Na2Os | |
| Molar mass | 448.91 g·mol−1 |
| Appearance | red solid |
| Density | 3.221 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
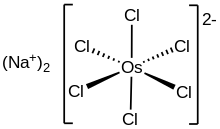
Sodium hexachloroosmate is the inorganic compound with the formula Na
2OsCl
6. A red solid, it is the disodium salt of the osmium(VI) complex [OsCl
6]2−. The anion is an octahedral complex with Os-Cl distance of 2.325(3) Å, as established by X-ray crystallography.[1] The compound can be prepared by reaction of a suspension of osmium metal in molten sodium chloride with chlorine:[2]
- Os + 2 NaCl + 2 Cl
2 → Na
2OsCl
6
Hexachloroosmate is paramagnetic, with a low-spin d2 configuration.
References
- ↑ Rudnitskaya, O. V.; Kultyshkina, E. K.; Dobrokhotova, E. V.; Tereshina, T. A.; Popova, A. S.; Zubavichus, Ya. V.; Khrustalev, V. N. (2019). "Crystal Structure of Na2[OsCl6]". Journal of Structural Chemistry 60 (7): 1086–1090. doi:10.1134/S0022476619070096.
- ↑ H. L. Grube (1963). "Sodium Hexachloroosmate(VI)". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 2pages=1602. NY, NY: Academic Press.
 |

