Chemistry:Thorium oxalate
From HandWiki
| |
| Identifiers | |
|---|---|
| EC Number |
|
PubChem CID
|
|
| Properties | |
| C4O8Th | |
| Molar mass | 408.07 g/mol 444.114 g/mol (dihydrate) |
| Density | 4.637 g/cm3 (anhydrous) |
Solubility product (Ksp)
|
5.01 × 10−25 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
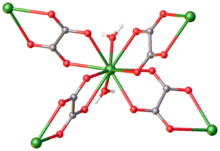
Thorium oxalate is the inorganic compound with the formula Th(C2O4)2(H2O)4. It is a white insoluble solid prepared by the reaction of thorium(IV) salts with an oxalic acid.[1] The material is a coordination polymer. Each Th(IV) center is bound to 10 oxygen centers: eight provided by the bridging oxalates and two by a pair of aquo ligands. Two additional water of hydration are observed in the lattice.[2]
The solubility product (Ksp) of thorium oxalate is 5.01 × 10−25.[3] Density of anhydrous thorium oxalate is 4.637 g/cm3.
References
- ↑ Enver Oktay, Ahmet Yayli (2001) Physical properties of thorium oxalate powders and their influence on the thermal decomposition Journal of Nuclear Materials Volume 288, Issue 1, January 2001, Pages 76–82
- ↑ Ziegelgruber, Kate L.; Knope, Karah E.; Frisch, Mark; Cahill, Christopher L. (2008). "Hydrothermal Chemistry of Th(IV) with Aromatic dicarboxylates: New Framework Compounds and in Situ Ligand Syntheses". Journal of Solid State Chemistry 181 (2): 373–381. doi:10.1016/j.jssc.2007.12.008. Bibcode: 2008JSSCh.181..373Z.
- ↑ Taishi Kobayashi, Takayuki Sasaki, Ikuji Takagi, Hirotake Moriyama (2009) Solubility of Thorium(IV) in the Presence of Oxalic and Malonic Acids Journal of Nuclear Science and Technology, Vol. 46, No. 11, p. 1085–1090
External links
- Atomistry.com: Thorium oxalate info page
- International Bio-Analytical Industries: Thorium Oxalate Dihydrate
 |

