Chemistry:Thorium(IV) chloride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| ThCl4 | |
| Molar mass | 373.849 g/mol |
| Appearance | white needles hygroscopic |
| Density | 4.59 g/cm3, solid |
| Melting point | 770 °C (1,420 °F; 1,040 K) |
| Boiling point | 921 °C (1,690 °F; 1,194 K) |
| Structure | |
| tetragonal | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
332 mg/kg intraperitoneal mouse |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thorium(IV) chloride describes a family of inorganic compounds with the formula ThCl4(H2O)n. Both the anhydrous and tetrahydrate (n = 4) forms are known. They are hygroscopic, water-soluble white salts.
Structures
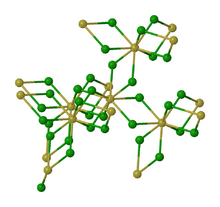
thumb|left|162px|Alternative view of the structure of solid ThCl4. The structure of thorium(IV) chloride features 8-coordinate Th centers with doubly bridging chloride ligands.[1]
Synthesis
ThCl4 was an intermediate in the original isolation of thorium metal by Jons Jacob Berzelius.[2]
Thorium(IV) chloride can be produced in a variety of ways. One method is a carbothermic reaction, 700 °C to 2600 °C, involving thorium oxides and carbon in a stream of chlorine gas:
- ThO2 + 2 C + 4 Cl2 → ThCl4 + 2 CO
The chlorination reaction can be effected with carbon tetrachloride:[3][4]
- Th(C2O4)2 + CCl4 → ThCl4 + 3 CO + 3 CO2
In another two-step method, thorium metal reacts with ammonium chloride:
- Th + 6 NH4Cl → (NH4)2ThCl6 + 4 NH3 + 2 H2
The hexachloride salt is then heated at 350 °C under a high vacuum to produce ThCl4.[5]
Reactions
- Lewis base adducts
ThCl4 reacts with Lewis bases to give molecular adducts, such as ThCl4(DME)2 and ThCl4(TMEDA)2.[5]
- Reduction to Th metal
Thorium(IV) chloride is an intermediate in the purification of thorium, which can be affected by:
- Reduction of ThCl4 with alkali metals.
- Electrolysis of anhydrous thorium(IV) chloride in fused mixture of NaCl and KCl.
- Ca reduction of a mixture of ThCl4 with anhydrous zinc chloride.[6]
References
- ↑ Mason, J. T.; Jha, M. C.; Chiotti, P. (1974). "Crystal Structures of ThCl4 Polymorphs". Journal of the Less Common Metals 34: 143–151. doi:10.1016/0022-5088(74)90224-0.
- ↑ Weeks, Mary Elvira (1932-07-01). "The Discovery of the Elements. XI. Some Elements Isolated with the Aid of Potassium and Sodium: Zirconium, Titanium, Cerium, and Thorium". Journal of Chemical Education 9 (7): 1231. doi:10.1021/ed009p1231. ISSN 0021-9584. Bibcode: 1932JChEd...9.1231W.
- ↑ Brauer, Georg (1963). Handbook Of Preparative Inorganic Chemistry. New York: Academic Press.
- ↑ Gutierrez, R. L.; Herbst, R. J. (October 1979). "Preliminary Fabrication Studies of Alternative LMFBR Carbide Fuels". Los Alamos Scientific Laboratory. doi:10.2172/5688597. http://www.osti.gov/scitech/servlets/purl/5688597-2bGijb/.
- ↑ 5.0 5.1 Cantat, Thibault; Scott, Brian L.; Kiplinger, Jaqueline L. (2010-01-25). "Convenient Access to the Anhydrous Thorium Tetrachloride Complexes ThCl4(DME)2, ThCl4(1,4-dioxane)2 and ThCl4(THF)3.5 using Commercially Available and Inexpensive Starting Materials" (in en). Chemical Communications 46 (6): 919–21. doi:10.1039/b923558b. ISSN 1364-548X. PMID 20107650. https://zenodo.org/record/1229998.
- ↑ "Periodic Table of Elements: Los Alamos National Laboratory". http://periodic.lanl.gov/90.shtml.
 |

