Chemistry:Helenalin
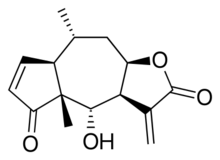
| |
| Names | |
|---|---|
| IUPAC name
(8αH)-6α-Hydroxy-4-oxo-10α-ambrosa-2,11(13)-dieno-12,8-lactone
| |
| Systematic IUPAC name
(3aS,4S,4aR,7aR,8R,9aR)-4-Hydroxy-4a,8-dimethyl-3-methylidene-3,3a,4,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H18O4 | |
| Molar mass | 262.305 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.[1][2]
Structure and reactivity
Helenalin belongs to the group of sesquiterpene lactones which are characterised by a lactone ring. Beside this ring, the structure of helenalin has two reactive groups (α-methylene-γ-butyrolactone and a cyclopentenone group) that can undergo a Michael addition.[3][4] The double bond in the carbonyl group can undergo a Michael addition with a thiol group, also called a sulfhydryl group. Therefore, helenalin can interact with proteins by forming covalent bonds to the thiol groups of cysteine-containing proteins/peptides, such as glutathione. This effect can disrupt the molecule's biological function.[2] Addition reactions can occur because thiol groups are strong nucleophiles; a thiol has a lone pair of electrons.[5]
Chemical derivatives
There are several derivatives of helenaline known within the same sesquiterpene lactone group; pseudoguaianolides. Most of these derivatives occur naturally, such as the compound dihydrohelenalin, but there are also some semi-synthetic derivatives known, such as 2β-(S-glutathionyl)-2,3-dihydrohelenalin.[1][2] In general, most derivatives are more toxic than helenalin itself. Among these, derivatives with the shortest ester groups are most likely to contain a higher toxicity.[6] Other derivatives include 11α,13-dihydrohelenalin acetate, 2,3-dehydrohelenalin and 6-O-isobutyrylhelenalin. The molecular conformation differs between helenalin and its derivatives, which affects the lipophilicity and the accessibility of the Michael addition sites. Poorer accessibility results in a compounds with lower toxicity.[citation needed] Another possibility is that a derivative lacking one of the reactive groups, such as the cyclopentenone group, may have a lower toxicity.[citation needed]
Some biochemical effects of helenalin
Helenalin can target the p65 subunit (also called RelA) of the transcription factor NF-κB. It can react with Cys38 in RelA by Michael addition. Both reactive groups, α-methylene-γ-butyrolactone and cyclopentene, can react with this cysteine.[3] It was also found that helenalin can inhibit human telomerase, a ribonucleoprotein complex, by Michael addition. In this case also, both reactive groups of helenalin can interact with the thiol group of a cysteine and inhibit the telomerase activity.[7] Helenalin inhibits the formation of leukotrienes in human blood cells by inhibiting LTC4 synthase activity. Helenalin reacts with its cyclopentenone ring to the thiol group of the synthase.[2]
Metabolism
Helenalin inhibits cytochrome P450 enzymes by reacting with thiol groups, resulting in inhibition of the mixed-function oxidase system. These effects are important for the cytotoxicity of helenalin. The levels of glutathione, which contains sulfhydryl groups, are reduced in helenaline-treated cells, further increasing the toxicity of helenalin. Depending on the dose of helenalin, thiol-bearing compounds such as glutathione may provide some protection to cells from helenalin toxicity. It was also seen that helenalin increase CPK and LDH activities in serum and that it inhibits multiple enzymes of the liver involved in triglyceride synthesis. Therefore, helenaline causes acute liver toxicity, accompanied by a decrease in cholesterol levels.[8]
Helenalin also suppresses essential immune functions, such as those mediated by activated CD4+ T-cells, by multiple mechanisms.[9]
In vitro anti-inflammatory and anti-neoplastic effects
Helenalin and some of its derivatives have been shown to have potent anti-inflammatory and anti-neoplastic effects in vitro. Some studies have suggested that the inhibition by helenalin of platelet leukotriene C4 synthase, telomerase activity and transcription factor NF-κB contributes to helenalin's in vitro anti-inflammatory and anti-neoplastic activity[2][7][10] .[11][12] The dose used varied per study. There is currently no in vivo evidence regarding helenalin's anti-inflammatory and anti-tumour effects, if any. The efficacy of helenalin for treatment of pain and swelling, when applied topically, is not supported by the current available evidence at doses of 10% or lower. For doses higher that 10%, more research is required whether those remain safe and are more efficient than the current available medications.[13]
Application
In former times, plant extracts containing helenalin were used as a herbal medicine for the treatment of sprains, blood clots, muscle strain and rheumatic complaints.[9] Currently helenalin is used topically in homeopathic gels and microemulsions. Helenalin is not FDA-approved for medical application.[14]
Toxicity
When applied topically on humans, helenalin can cause contact dermatitis in sensitive individuals. However, it is considered generally safe when applied this way. Oral administration of large doses of helenalin can cause gastroenteritis, muscle paralysis, and cardiac and liver damage. The toxicity of helenalin was studied in mammalian species such as mice, rat, rabbit and sheep, where the oral LD50 of helenalin was established between 85 and 150 mg/kg.[15][16] It was shown in a mouse model that helenalin caused reduced levels of cholesterol. In a rat model, alcohol hepatic injury was prevented by helenalin administration.[8][17] Parenteral administration showed a higher toxic effect when compared to oral administration.[18][19]
Pharmacology
Helenalin has a variety of observed effects in vitro including anti-inflammatory and antitumour activities.[20] Helenalin has been shown to selectively inhibit the transcription factor NF-κB, which plays a key role in regulating immune response, through a unique mechanism.[21] In vitro, it is also a potent, selective inhibitor of human telomerase[7]—which may partially account for its antitumor effects—has anti-trypanosomal activity,[22][23] and is toxic to Plasmodium falciparum.[24]
Animal and in vitro studies have also suggested that helenalin can reduce the growth of Staphylococcus aureus and reduce the severity of S. aureus infection.[25]
References
- ↑ 1.0 1.1 "Sesquiterpene lactones in Arnica montana: helenalin and dihydrohelenalin chemotypes in Spain". Planta Medica 75 (6): 660–6. May 2009. doi:10.1055/s-0029-1185362. PMID 19235681.
- ↑ 2.0 2.1 2.2 2.3 2.4 Tornhamre, Susanne; Schmidt, Thomas J.; Näsman-Glaser, Barbro; Ericsson, Inger; Lindgren, Jan Åke (2001). "Inhibitory effects of helenalin and related compounds on 5-lipoxygenase and leukotriene C 4 synthase in human blood cells". Biochemical Pharmacology 62 (7): 903–911. doi:10.1016/S0006-2952(01)00729-8. PMID 11543725.
- ↑ 3.0 3.1 "Helenalin Analogues Targeting NF-κB p65: Thiol Reactivity and Cellular Potency Studies of Varied Electrophiles". ChemMedChem 13 (4): 303–311. February 2018. doi:10.1002/cmdc.201700752. PMID 29349898.
- ↑ "Differential effects of Helenalin, an anti-inflammatory sesquiterpene lactone, on the proteome, metabolome and the oxidative stress response in several immune cell types". Toxicology in Vitro 40: 45–54. April 2017. doi:10.1016/j.tiv.2016.12.010. PMID 27998807.
- ↑ "The basics of thiols and cysteines in redox biology and chemistry". Free Radical Biology & Medicine 80: 148–57. March 2015. doi:10.1016/j.freeradbiomed.2014.11.013. PMID 25433365.
- ↑ "Structure-cytotoxicity relationships of some helenanolide-type sesquiterpene lactones". Journal of Natural Products 60 (3): 252–7. March 1997. doi:10.1021/np960517h. PMID 9090867.
- ↑ 7.0 7.1 7.2 "Potent inhibition of human telomerase by helenalin". Cancer Letters 227 (2): 169–74. September 2005. doi:10.1016/j.canlet.2004.11.045. PMID 16112419.
- ↑ 8.0 8.1 "Acute toxicity of helenalin in BDF1 mice". Fundamental and Applied Toxicology 10 (2): 302–12. February 1988. doi:10.1016/0272-0590(88)90315-6. PMID 3356317.
- ↑ 9.0 9.1 "Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms". Molecular Immunology 46 (15): 2892–901. September 2009. doi:10.1016/j.molimm.2009.07.004. PMID 19656571.
- ↑ "Mode of action of sesquiterpene lactones as anti-inflammatory agents". Journal of Pharmaceutical Sciences 69 (5): 537–43. May 1980. doi:10.1002/jps.2600690516. PMID 6247478.
- ↑ "Sesquiterpene antitumor agents: inhibitors of cellular metabolism". Science 196 (4289): 533–6. April 1977. doi:10.1126/science.191909. PMID 191909. Bibcode: 1977Sci...196..533L.
- ↑ "Helenalin and 11 alpha,13-dihydrohelenalin, two constituents from Arnica montana L., inhibit human platelet function via thiol-dependent pathways". Thrombosis Research 57 (6): 839–45. March 1990. doi:10.1016/0049-3848(90)90151-2. PMID 2116680.
- ↑ Brito, N; Knipschild, P; Doreste-Alonso, J (2014). "Systematic Review on the Efficacy of Topical Arnica montanafor the Treatment of Pain, Swelling and Bruises". Journal of Musculoskeletal Pain 22 (2): 216–223. doi:10.3109/10582452.2014.883012.
- ↑ "U.S. National Library of Medicine," [Online]. Available: https://clinicaltrials.gov/ct2/results?cond=&term=arnica+montana&cntry=&state=&city=&dist=. [Accessed 8 March 2018].
- ↑ "Antihyperlipidemic activity of sesquiterpene lactones and related compounds". Journal of Pharmaceutical Sciences 69 (6): 694–7. June 1980. doi:10.1002/jps.2600690622. PMID 7205585.
- ↑ "Mammalian toxicity of helenalin, the toxic principle of Helenium microcephalum CD (smallhead sneezeweed)". American Journal of Veterinary Research 37 (7): 859–61. July 1976. PMID 937811.
- ↑ "Helenalin attenuates alcohol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and suppressing HSC activation". Fitoterapia 95: 203–13. June 2014. doi:10.1016/j.fitote.2014.03.020. PMID 24704336.
- ↑ B. H. Rumack, "POISINDEX(R) Information System Micromedex, Inc.", CCIS, vol. 172, 2017.
- ↑ A. H. Hall and B. H. Rumack, "TOMES(R) Information System Micromedex, Inc." CCIS, vol. 172, 2017
- ↑ "Increased intracellular Ca2+ signaling caused by the antitumor agent helenalin and its analogues". Cancer Chemotherapy and Pharmacology 34 (4): 344–50. 1994. doi:10.1007/BF00686043. PMID 8033301.
- ↑ "The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65". The Journal of Biological Chemistry 273 (50): 33508–16. December 1998. doi:10.1074/jbc.273.50.33508. PMID 9837931.
- ↑ "The trypanocidal effect of sesquiterpene lactones helenalin and mexicanin on cultured epimastigotes". The Journal of Parasitology 91 (1): 170–4. February 2005. doi:10.1645/GE-3373. PMID 15856894.
- ↑ "Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones". Planta Medica 68 (8): 750–1. August 2002. doi:10.1055/s-2002-33799. PMID 12221603.
- ↑ "Pseudoguaianolide sesquiterpene lactones with high activities against the human malaria parasite Plasmodium falciparum". Phytotherapy Research 18 (2): 184–6. February 2004. doi:10.1002/ptr.1376. PMID 15022176.
- ↑ "Helenalin reduces Staphylococcus aureus infection in vitro and in vivo". Veterinary Microbiology 119 (2–4): 330–8. January 2007. doi:10.1016/j.vetmic.2006.08.020. PMID 17010538.
 |

