Chemistry:Sesquiterpene lactone
Sesquiterpene lactones (SLs) are a class of sesquiterpenoids that contain a lactone ring. They are most often found in plants of the family Asteraceae (daisies, asters). Other plant families with SLs are Umbelliferae (celery, parsley, carrots) and Magnoliaceae (magnolias). A collection of colorless, lipophilic solids, SLs are a rich source of drugs.[1] They can be allergenic and toxic in grazing livestock[2] causing severe neurological problems in horses. Some are also found in corals such as Maasella edwardsi.
Types
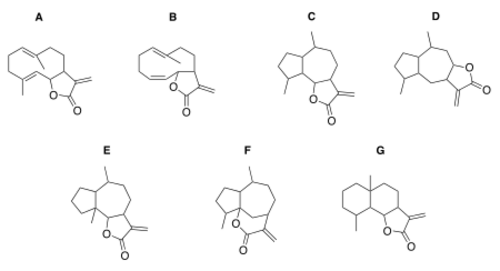
A: Germacranolides, B: Heliangolides, C+D: Guaianolides, E: Pseudoguaianolides, F: Hypocretenolides, G: Eudesmanolides.
Sesquiterpene lactones can be divided into several main classes including germacranolides, heliangolides, guaianolides, pseudoguaianolides, hypocretenolides, and eudesmanolides.
Examples
Artemisinin, a new, highly-effective anti-malarial compound, is a sesquiterpene lactone found in Artemisia annua. Lactucin, desoxylactucin, lactucopicrin, lactucin-15-oxalate, lactucopicrin-15-oxalate are some of the most prominent found in lettuce and spinach, giving most of the bitter taste to these crops.
One eudesmanolide, 3-oxo-5αH,8βH-eudesma-1,4(15),7(11)-trien-8,12-olide, can work with vernolic acid and other compounds in plants to reduce inflammation.[3]
Sesquiterpene lactone-containing plants
Some plants containing these compounds include:
- Artichoke
- Boneset Eupatorium perfoliatum[4]
- Burdock
- Calea ternifolia
- Chamomile
- Chrysanthemum
- Cocklebur
- Feverfew
- Gaillardia
- Ginkgo biloba
- Laurus nobilis[5]
- Lettuce (Lactuca)
- Marsh elder
- Mugwort
- Parthenium
- Poverty weed
- Pyrethrum
- Ragweed
- Sagebrush
- Sneezeweed
- Spinach
- Star anise
- Sunflower
- Ironweed[6]
- Wormwood
- Yellow star thistle
- bitter leaf
Quorum sensing inhibitors
Sesquiterpene lactones have been found to possess the ability to inhibit quorum sensing in bacteria.[7]
References
- ↑ Ghantous, Akram; Gali-Muhtasib, Hala; Vuorela, Heikki; Saliba, Najat A.; Darwiche, Nadine (2010). "What Made Sesquiterpene Lactones Reach Cancer Clinical Trials?". Drug Discovery Today 15 (15–16): 668–678. doi:10.1016/0305-1978(86)90101-8. PMID 20541036.
- ↑ "Sesquiterpene Lactones and their toxicity to livestock". Cornell University. http://poisonousplants.ansci.cornell.edu/toxicagents/sesqlactone/sesqlactone.html.
- ↑ "Sesquiterpene lactone suppresses vascular smooth muscle cell proliferation and migration via inhibition of cell cycle progression". Biol. Pharm. Bull. 30 (9): 1754–7. September 2007. doi:10.1248/bpb.30.1754. PMID 17827734.
- ↑ "Sesquiterpene lactones of Eupatorium perfoliatum". J. Org. Chem. 42 (13): 2264–71. June 1977. doi:10.1021/jo00433a017. PMID 874606.
- ↑ "Two new sesquiterpene lactones from the leaves of Laurus nobilis". Chem. Pharm. Bull. 54 (8): 1187–9. August 2006. doi:10.1248/cpb.54.1187. PMID 16880666.
- ↑ Herbal Medicine Past and Present: A reference guide to medicinal plants. Duke University Press. 1990. pp. 265–. ISBN 0-8223-1019-8. https://books.google.com/books?id=0JaqB07uTx4C&pg=PA265.
- ↑ "Inhibition of quorum sensing in Pseudomonas aeruginosa by sesquiterpene lactones". Phytomedicine 19 (13): 1173–7. October 2012. doi:10.1016/j.phymed.2012.07.003. PMID 22925726.
External links
 |

