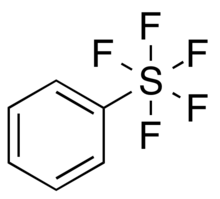
Chemistry:Pentafluorosulfanylbenzene
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentafluoro(phenyl)-λ6-sulfane | |
| Other names
Phenylsulfur pentafluoride, PhSF5
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H5F5S | |
| Molar mass | 204.16 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.49 g/mL |
| Boiling point | 149 °C (300 °F; 422 K)[1] |
| log P | 3.36 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H302, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P270, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P321, P330, P362+364Script error: No such module "Preview warning".Category:GHS errors, P370+378, P403+233, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentafluorosulfanylbenzene, or phenylsulfur pentafluoride, is an organosulfur compound with the formula C6H5SF5. It is colorless liquid with high chemical stability.
Reactivity
Pentafluorosulfanylbenzene possesses high chemical stability under a wide range of conditions including oxidizing, reducing, strongly acidic and strongly basic environments. For example, it does not react with a refluxing solution of sodium hydroxide in aqueous ethanol, but it can react with concentrated sulfuric acid at elevated temperatures. The pentafluorosulfanyl group is a strong electron withdrawing group, and leads to electrophilic aromatic substitution reactions at the meta position.[2]
Synthesis
Pentafluorosulfanylbenzene was originally synthesized by fluorination of diphenyl disulfide by AgF2, a method that suffers from low yield. The best known method of synthesis is the fluorination of diphenyl disulfide with xenon difluoride, but it still only has a 25% yield.[3]
[math]\ce{ C12H10S2 + 5 XeF2 -> 2C6H5SF5 + 5Xe }[/math]
References
- ↑ Sheppard, William A. (1960-09-01). "Arylsulfur Trifluorides and Pentafluorides". Journal of the American Chemical Society 82 (17): 4751–4752. doi:10.1021/ja01502a083. ISSN 0002-7863.
- ↑ Sheppard, William A. (1962-08-01). "Arylsulfur Pentafluorides". Journal of the American Chemical Society 84 (16): 3064–3072. doi:10.1021/ja00875a006. ISSN 0002-7863.
- ↑ Sergeeva, Tatiana A.; Dolbier, William R. (2004-07-01). "A New Synthesis of Pentafluorosulfanylbenzene" (in en). Organic Letters 6 (14): 2417–2419. doi:10.1021/ol0491991. ISSN 1523-7060. PMID 15228293. https://pubs.acs.org/doi/10.1021/ol0491991.
 |

