Chemistry:Bactoprenol
| |
| Names | |
|---|---|
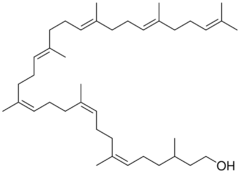
| IUPAC name
(6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-6,10,14,18,22,26,30,34,38,42-decaen-1-ol
| |
| Other names
Dolichol-11
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C55H92O | |
| Molar mass | 769.318 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bactoprenol also known as dolichol-11 and (isomerically vaguely) C55-isoprenyl alcohol (C55-OH) is a lipid first identified in certain species of lactobacilli.[1] It is a hydrophobic alcohol that plays a key role in the growth of cell walls (peptidoglycan) in Gram-positive bacteria.[2]
The double bonds all have the Z configuration except for the three ω-terminal ones, which are biosynthetically derived from (E,E)-farnesyl diphosphate.[3]
Occurrence
Bactoprenol is a lipid synthesized from mevalonic acid and is the most abundant lipid found in certain species of lactobacilli.[1] Bactoprenol is found in both mesosomal and plasma membranes.[4] Mesosomal and plasma bactoprenol are synthesized independently from each other.[5]
Function
Bactoprenol is thought to play a key role in the formation of cell walls in gram-positive bacteria by cycling peptidoglycan monomers through the plasma membrane and inserting these monomers at points of growth in the bacterial cell wall.[6]
Antibiotic significance
Because bactoprenol is so important for cell growth, numerous antibiotic compounds function by disrupting the bactoprenol-mediated transportation pathway.[7] This strategy was first identified by studying the antibiotic mechanism of friulimicin B.[8] Since then, other antibiotics that make use of a similar mechanism have been identified, including nisin[9] and lantibiotics such as NAI-107.[10]
References
- ↑ 1.0 1.1 "The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid". The Biochemical Journal 99 (1): 123–7. April 1966. doi:10.1042/bj0990123. PMID 5965329.
- ↑ Kaiser, Gary (September 2018). "BIOL 230 Lecture Guide - Synthesis of Peptidoglycan - Role of Bactoprenol". http://faculty.ccbcmd.edu/courses/bio141/lecguide/unit2/control/bactopren_ill_02.html.
- ↑ "Synthesis and characterization of dolichols and polyprenols of designed geometry and chain length.". Chemistry and Physics of Lipids 51 (3–4): 159–70. November 1989. doi:10.1016/0009-3084(89)90003-0.
- ↑ "Spheroplasts of Lactobacillus casei and the cellular distribution of bactoprenol". Journal of Cell Science 7 (3): 755–85. November 1970. doi:10.1242/jcs.7.3.755. PMID 4250091.
- ↑ "The occurrence of bactoprenol in the mesosome and plasma membranes of Lactobacillus casei and Lactobacillus plantarum". Journal of General Microbiology 70 (1): 87–98. April 1972. doi:10.1099/00221287-70-1-87. PMID 4625239.
- ↑ Kaiser, Gary (September 2018). "BIOL 230 Lecture Guide - Synthesis of Peptidoglycan - Role of Bactoprenol". http://faculty.ccbcmd.edu/courses/bio141/lecguide/unit2/control/bactopren_ill_02.html.
- ↑ "Lipid II and other bactoprenol-bound cell wall precursors as drug targets". Current Opinion in Investigational Drugs 11 (2): 157–64. February 2010. PMID 20112165.
- ↑ "The lipopeptide antibiotic Friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate". Antimicrobial Agents and Chemotherapy 53 (4): 1610–8. April 2009. doi:10.1128/AAC.01040-08. PMID 19164139.
- ↑ "Aggregates of nisin with various bactoprenol-containing cell wall precursors differ in size and membrane permeation capacity". Biochimica et Biophysica Acta (BBA) - Biomembranes 1828 (11): 2628–36. November 2013. doi:10.1016/j.bbamem.2013.07.014. PMID 23872123.
- ↑ "The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions". The Journal of Biological Chemistry 289 (17): 12063–76. April 2014. doi:10.1074/jbc.M113.537449. PMID 24627484.
 |

