Biology:Lactobacillus
| Lactobacillus | |
|---|---|
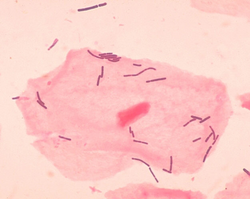
| |
| Lactobacillus sp. near a squamous epithelial cell | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Lactobacillales |
| Family: | Lactobacillaceae |
| Genus: | Lactobacillus Beijerinck 1901 (Approved Lists 1980)[1] |
| Type species | |
| Lactobacillus delbrueckii (Leichmann 1896) Beijerinck 1927 (Approved Lists 1980)[1]
| |
| Species | |
|
See text | |
Lactobacillus is a genus of gram-positive, aerotolerant anaerobes or microaerophilic, rod-shaped, non-spore-forming bacteria.[2][3] Until 2020, the genus Lactobacillus comprised over 260 phylogenetically, ecologically, and metabolically diverse species; a taxonomic revision of the genus assigned lactobacilli to 25 genera (see § Taxonomy below).[3]
Lactobacillus species constitute a significant component of the human and animal microbiota at a number of body sites, such as the digestive system, and the female genital system.[4] In women of European ancestry, Lactobacillus species are normally a major part of the vaginal microbiota.[5][6] Lactobacillus forms biofilms in the vaginal and gut microbiota,[7] allowing them to persist during harsh environmental conditions and maintain ample populations.[8] Lactobacillus exhibits a mutualistic relationship with the human body, as it protects the host against potential invasions by pathogens, and in turn, the host provides a source of nutrients.[9] Lactobacilli are among the most common probiotic found in food such as yogurt, and it is diverse in its application to maintain human well-being, as it can help treat diarrhea, vaginal infections, and skin disorders such as eczema.[10]
Metabolism
Lactobacilli are homofermentative, i.e. hexoses are metabolised by glycolysis to lactate as major end product, or heterofermentative, i.e. hexoses are metabolised by the Phosphoketolase pathway to lactate, CO2 and acetate or ethanol as major end products.[11] Most lactobacilli are aerotolerant and some species respire if heme and menaquinone are present in the growth medium.[11] Aerotolerance of lactobacilli is manganese-dependent and has been explored (and explained) in Lactiplantibacillus plantarum (previously Lactobacillus plantarum).[12] Lactobacilli generally do not require iron for growth.[13]
The Lactobacillaceae are the only family of the lactic acid bacteria (LAB) that includes homofermentative and heterofermentative organisms; in the Lactobacillaceae, homofermentative or heterofermentative metabolism is shared by all strains of a genus.[3][11] Lactobacillus species are all homofermentative, do not express pyruvate formate lyase, and most species do not ferment pentoses.[3][11] In L. crispatus, pentose metabolism is strain specific and acquired by lateral gene transfer.[14] {{Annotated image 4 | image = Microbiota-derived 3-Indolepropionic acid-notext.svg | link = Commons:File:Microbiota-derived 3-Indolepropionic acid.svg | header = Tryptophan metabolism by human gastrointestinal microbiota ( ) | header_align = center | header_background = #F0F8FF | align = left | image-width = 600 | image-left = 0 | image-top = 10 | width = 580 | height = 470 | alt = Tryptophan metabolism diagram | caption = {{{caption|This diagram shows the biosynthesis of bioactive compounds (indole and certain other derivatives) from by bacteria in the gut.[15] Indole is produced from tryptophan by bacteria that express tryptophanase.[15] Clostridium sporogenes metabolizes tryptophan into indole and subsequently 3-indolepropionic acid (IPA),[16] a highly potent neuroprotective antioxidant that scavenges hydroxyl radicals.[15][17][18] IPA binds to the pregnane X receptor (PXR) in intestinal cells, thereby facilitating mucosal homeostasis and barrier function.[15] Following absorption from the intestine and distribution to the brain, IPA confers a neuroprotective effect against cerebral ischemia and Alzheimer's disease.[15] Lactobacillus species metabolize tryptophan into {{when pagename is|Indole-3-carboxaldehyde=indole-3-carboxaldehyde|other=indole-3-aldehyde}} (I3A) which acts on the aryl hydrocarbon receptor (AhR) in intestinal immune cells, in turn increasing interleukin-22 (IL-22) production.[15] Indole itself triggers the secretion of glucagon-like peptide-1 (GLP-1) in intestinal L cells and acts as a ligand for AhR.[15] Indole can also be metabolized by the liver into ]], a compound that is toxic in high concentrations and associated with vascular disease and renal dysfunction.[15] AST-120 (activated charcoal), an intestinal sorbent that is [[Oral administrat[[Physics:taken by mouth,Chemistry:Adsorption|adsorbs i]]ndole, in turn decreasing the concentration of indoxyl sulfate in blood plasma.[15] }}} | annot-font-size = 14 | annot-text-align = left | annotations =
expressing
bacteria
immune
cells
↓Activation of glial cells and astrocytes
↓4-Hydroxy-2-nonenal levels
↓DNA damage
–Antioxidant
–Inhibits β-amyloid fibril formation
↑IL-22 production
↑Oxidative stress
↑Smooth muscle cell proliferation
↑Aortic wall thickness and calcification
↑Renal dysfunction
–Uremic toxin
}}
Genomes
The genomes of lactobacilli are highly variable, ranging in size from 1.2 to 4.9 Mb (megabases).[3] Accordingly, the number of protein-coding genes ranges from 1,267 to about 4,758 genes (in Fructilactobacillus sanfranciscensis and Lentilactobacillus parakefiri, respectively).[19][20] Even within a single species there can be substantial variation. For instance, strains of L. crispatus have genome sizes ranging from 1.83 to 2.7 Mb, or 1,839 to 2,688 open reading frames.[21] Lactobacillus contains a wealth of compound microsatellites in the coding region of the genome, which are imperfect and have variant motifs.[22] Many lactobacilli also contain multiple plasmids. A recent study has revealed that plasmids encode the genes which are required for adaptation of lactobacilli to the given environment.[23]
Species
The genus Lactobacillus comprises the following species:[24][25]
- Lactobacillus acetotolerans Entani et al. 1986
- Lactobacillus acidophilus (Moro 1900) Hansen and Mocquot 1970 (Approved Lists 1980)
- "Lactobacillus alvi" Kim et al. 2011
- Lactobacillus amylolyticus Bohak et al. 1999
- Lactobacillus amylovorus Nakamura 1981
- Lactobacillus apis Killer et al. 2014
- "Lactobacillus backi" Bohak et al. 2006
- Lactobacillus bombicola Praet et al. 2015
- Lactobacillus colini Zhang et al. 2017
- Lactobacillus crispatus (Brygoo and Aladame 1953) Moore and Holdeman 1970 (Approved Lists 1980)
- Lactobacillus delbrueckii (Leichmann 1896) Beijerinck 1901 (Approved Lists 1980)
- Lactobacillus equicursoris Morita et al. 2010
- Lactobacillus fornicalis Dicks et al. 2000
- Lactobacillus gallinarum Fujisawa et al. 1992
- Lactobacillus gasseri Lauer and Kandler 1980
- Lactobacillus gigeriorum Cousin et al. 2012
- "Lactobacillus ginsenosidimutans" Jung et al. 2013
- Lactobacillus hamsteri Mitsuoka and Fujisawa 1988
- Lactobacillus helsingborgensis Olofsson et al. 2014
- Lactobacillus helveticus (Orla-Jensen 1919) Bergey et al. 1925 (Approved Lists 1980)
- Lactobacillus hominis Cousin et al. 2013
- Lactobacillus iners Falsen et al. 1999
- Lactobacillus intestinalis (ex Hemme 1974) Fujisawa et al. 1990
- Lactobacillus jensenii Gasser et al. 1970 (Approved Lists 1980)
- "Lactobacillus jinshani" Yu et al. 2020
- Lactobacillus johnsonii Fujisawa et al. 1992
- Lactobacillus kalixensis Roos et al. 2005
- Lactobacillus kefiranofaciens Fujisawa et al. 1988
- Lactobacillus kimbladii Olofsson et al. 2014
- Lactobacillus kitasatonis Mukai et al. 2003
- Lactobacillus kullabergensis Olofsson et al. 2014
- Lactobacillus melliventris Olofsson et al. 2014
- Lactobacillus mulieris Rocha et al. 2020
- Lactobacillus nasalidis Suzuki-Hashido et al. 2021
- Lactobacillus panisapium Wang et al. 2018
- Lactobacillus paragasseri Tanizawa et al. 2018
- Lactobacillus pasteurii Cousin et al. 2013
- Lactobacillus porci Kim et al. 2018
- Lactobacillus psittaci Lawson et al. 2001
- "Lactobacillus raoultii" Nicaise et al. 2018
- Lactobacillus rodentium Killer et al. 2014
- Lactobacillus rogosae Holdeman and Moore 1974 (Approved Lists 1980)
- Lactobacillus taiwanensis Wang et al. 2009
- "Lactobacillus thermophilus" Ayers and Johnson 1924
- "Lactobacillus timonensis" Afouda et al. 2017
- Lactobacillus ultunensis Roos et al. 2005
- Lactobacillus xujianguonis Meng et al. 2020
Taxonomy
The genus Lactobacillus currently contains 44 species which are adapted to vertebrate hosts or to insects.[3] In recent years, other members of the genus Lactobacillus (formerly known as the Leuconostoc branch of Lactobacillus) have been reclassified into the genera Atopobium, Carnobacterium, Weissella, Oenococcus, and Leuconostoc. The Pediococcus species P. dextrinicus has been reclassified as a Lapidilactobacillus dextrinicus [3][26] and most lactobacilli were assigned to Paralactobacillus or one of the 23 novel genera of the Lactobacillaceae.[3] Two websites inform on the assignment of species to the novel genera or species (http://www.lactobacillus.uantwerpen.be/; http://www.lactobacillus.ualberta.ca/).
| Genus | Meaning of the genus name | Properties of the genus |
|---|---|---|
| Lactobacillus | Rod-shaped bacillus from milk | Type species: L. delbrueckii.
Homofermentative with strain-specific ability to ferment pentoses, thermophilic, vancomycin-sensitive, adapted to vertebrate or insect hosts. |
| Holzapfelia | Wilhelm Holzapfel's lactobacilli | Type species: H. floricola.
Homofermentative, vancomycin sensitive, unknown ecology but likely host-adapted. |
| Amylolactobacillus | Starch-degrading lactobacilli | Type species: A. amylophilus.
Homofermentative, vancomycin sensitive, extracellular amylases are frequent, unknown ecology but likely host-adapted. |
| Bombilactobacillus | Lactobacilli from bees and bumblebees | Type species: B. mellifer.
Homofermentative, thermophilic, vancomycin resistant, small genome size, adapted to bees and bumblebees |
| Companilactobacillus | Companion-lactobacillus, referring to them growing in association with other lactobacilli in cereal, meat and vegetable fermentations | Type species: C. alimentarius.
Homofermentative with strain- or species-specific ability to ferment pentoses, vancomycin resistant, unknown ecology, likely nomadic |
| Lapidilactobacillus | Lactobacilli from stones | Type species: L. concavus.
Homofermentative with strain- or species-specific ability to ferment pentoses, vancomycin resistant, unknown ecology. |
| Agrilactobacillus | Lactobacilli from fields | Type species: A. composti.
Homofermentative, aerotolerant and vancomycin resistant. Genome size, G+C content of the genome and the source of the two species suggest a free-living lifestyle of the genus. |
| Schleiferilactobacillus | Karl Heinz Schleifer’s lactobacilli | Type species: S. perolens.
Homofermentative, vancomycin resistant, aerotolerant. Schleiferilactobacillus spp. have a large genome size, ferment a wide range of carbohydrates, and spoil beer and dairy products by copious production of diacetyl. |
| Loigolactobacillus | (Food) spoiling lactobacilli | Type species: L. coryniformis.
Homofermentative, vancomycin resistant, mesophilic or psychrotrophic organisms. |
| Lacticaseibacillus | Lactobacilli related to cheese | Type species: L. casei.
Homofermentative, vancomycin resistant; many species ferment pentoses, and are resistant to oxidative stress. L. casei and related species have a nomadic lifestyle. |
| Latilactobacillus | Widespread lactobacilli | Type species: L. sakei.
Homofermentative, mesophilic free living and environmental lactobacilli. Many strains are psychrotrophic and grow below 8 °C. |
| Dellaglioa | Franco Dellaglio’s lactobacilli | Type species: D. algidus.
Homofermentative, vancomycin resistant, aerotolerant and psychrophilic. |
| Liquorilactobacillus | Lactobacilli from liquor or liquids | Type species: L. mali.
Homofermentative, vancomycin resistant, motile organisms growing in liquid, plant-associated habitats. Many liquorilactobacilli produce EPS from sucrose and degrade fructans with extracellular fructanases. |
| Ligilactobacillus | Uniting (host adapted) lactobacilli | Type species: L. salivarius.
Homofermentative, vancomycin resistant, most ligilactobacilli are host adapted and many strains are motile. Several strains of Ligilactobacillus express urease to withstand gastric acidity. |
| Lactiplantibacillus | Lactobacilli related to plants | Type species: L. plantarum.
Homofermentative, vancomycin resistant organisms with a nomadic lifestyle that ferment a wide range of carbohydrates; most species metabolise phenolic acids by esterase, decarboxylase and reductase activities. L. plantarum expresses pseudocatalase and nitrate reductase activities. |
| Furfurilactobacillus | Lactobacilli from bran | Type species: F. rossiae.
Heterofermentative, vancomycin resistant, with large genome size, broad metabolic potential and unknown ecology. |
| Paucilactobacillus | Lactobacilli fermenting few carbohydrates | Type species: P. vaccinostercus.
Heterofermentative, vancomycin resistant, mesophilic or psychrotrophic, aerotolerant, most strains ferment pentoses but not disaccharides. |
| Limosilactobacillus | Slimy (biofilm-forming) lactobacilli | Type species: L. fermentum.
Heterofermentative, thermophilic, vancomycin resistant with two exceptions, Limosilactobacillus species are vertebrate host adapted and generally form exopolysaccharides from sucrose to support biofilm formation in the upper intestine of animals. |
| Fructilactobacillus | Fructose-loving lactobacilli | Type species: F. fructivorans.
Heterofermentative, vancomycin resistant, mesophilic, aerotolerant, small genome size. Fructilactobacilli are adapted to narrow ecological niches that relate to insects, flowers, or both. |
| Acetilactobacillus | Lactobacilli from vinegar | Type species: A. jinshani.
Heterofermentative, vancomycin resistant, grow in the pH range of 3–5; fermenting disaccharides and sugar alcohols but few hexoses and no pentoses. |
| Apilactobacillus | Lactobacilli from bees | Type species: A. kunkeei.
Heterofermentative, vancomycin resistant, small genome size, fermenting only few carbohydrates, adapted to bees and/or flowers. |
| Levilactobacillus | (Dough)-leavening lactobacilli | Type species: L. brevis.
Heterofermentative, vancomycin resistant, mesophilic or psychrotrophic, metabolise agmatine, environmental or plant-associated lifestyle. |
| Secundilactobacillus | Second lactobacilli, growing after other organisms depleted hexoses | Type species: S. collinoides.
Heterofermentative, vancomycin resistant, mesophilic or psychrotrophic, environmental or plant-associated lifestyle. Adapted to hexose-depleted habitats, most strains do not reduce fructose to mannitol but metabolize agmatine and diols. |
| Lentilactobacillus | Slow (growing) lactobacilli | Type species: L. buchneri.
Heterofermentative, vancomycin resistant, mesophilic, fermenting a broad spectrum of carbohydrates. Most lentilactobacilli are environmental or plant-associated, metabolise agmatine and convert lactate and/or diols. L. senioris and L. kribbianus form an outgroup to the genus; both species were isolated from vertrebrates and may transition to a host-adapted lifestyle. |
Phylogeny
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature[24] and the phylogeny is based on whole-genome sequences.[3]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Human health
Vaginal tract
Lactobacillus s.s. species are considered "keystone species" in the vaginal flora of reproductive-age women. Most, but not all, healthy women have vaginal floras dominated by one of four species of Lactobacillus: L. iners, L. crispatus, L. gasseri and L. jensenii. Other women have a more diverse mix of anaerobic microorganisms.[5]
Interactions with pathogens
Lactobacilli produce hydrogen peroxide which inhibits the growth and virulence of the fungal pathogen Candida albicans in vitro and in vivo.[27][28] In vitro studies have also shown that lactobacilli reduce the pathogenicity of C. albicans through the production of organic acids and certain metabolites.[29] Both the presence of metabolites, such as sodium butyrate, and the decrease in environmental pH caused by the organic acids reduce the growth of hyphae in C. albicans, which reduces its pathogenicity.[29] Lactobacilli also reduce the pathogenicity of C. albicans by reducing C. albicans biofilm formation.[29] Biofilm formation is reduced by both the competition from lactobacilli, and the formation of defective biofilms which is linked to the reduced hypha growth mentioned earlier.[29] On the other hand, following antibiotic therapy, certain Candida species can suppress the regrowth of lactobacilli at body sites where they cohabitate, such as in the gastrointestinal tract.[27][28]
In addition to its effects on C. albicans, Lactobacillus sp. also interact with other pathogens. For example, Limosilactobacillus reuteri (formerly Lactobacillus reuteri) can inhibit the growth of many different bacterial species by using glycerol to produce the antimicrobial substance called reuterin.[30] Another example is Ligilactobacillus salivarius (formerly Lactobacillus salivarius), which interacts with many pathogens through the production of salivaricin B, a bacteriocin.[31]
Probiotics
Fermentive bacteria like lactic acid bacteria (LAB) produce hydrogen peroxide which protects themselves from oxygen toxicity. The accumulation of hydrogen peroxide in growth media, and its antagonistic effects on Staphylococcus aureus and Pseudomonas, have been demonstrated by researchers. LAB cultures have been used as starter cultures to create fermented foods since the beginning of the 20th century. Elie Metchnikoff won a Nobel prize in 1908 for his work on LAB.[32]
Lactobacilli administered in combination with other probiotics benefits cases of irritable bowel syndrome (IBS), although the extent of efficacy is still uncertain.[33] The probiotics help treat IBS by returning homeostasis when the gut microbiota experiences unusually high levels of opportunistic bacteria.[9] In addition, lactobacilli can be administered as probiotics during cases of infection by the ulcer-causing bacterium Helicobacter pylori.[34] Helicobacter pylori is linked to cancer, and antibiotic resistance impedes the success of current antibiotic-based eradication treatments.[34] When probiotic lactobacilli are administered along with the treatment as an adjuvant, its efficacy is substantially increased and side effects may be lessened.[34]
Gastroesophageal reflux disease (GERD) is a common condition associated with bile acid-induced oxidative stress and accumulation of reactive oxygen species (ROS) in esophageal tissues that cause inflammation and DNA damage.[35] In an experimental model of GERD, Lactobacillus species (L. acidophilus, L. plantarum and L. fermentum) facilitated the repair of DNA damage caused by bile-induced ROS.[35] For patients with GERD, there is significant interest in the anti-inflammatory effect of Lactobacilli that may help prevent progression to Barrett’s esophagus and esophageal adenocarcinoma.[35]
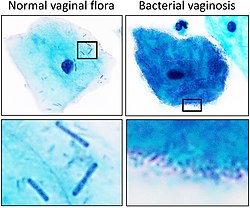
Also, lactobacilli are used to help control urogenital and vaginal infections, such as bacterial vaginosis (BV). Lactobacilli produce bacteriocins to suppress pathogenic growth of certain bacteria,[36] as well as lactic acid and H2O2 (hydrogen peroxide). Lactic acid lowers the vaginal pH to around 4.5 or less, hampering the survival of other bacteria, and H2O2 reestablishes the normal bacterial microbiota and normal vaginal pH.[36] In children, lactobacilli such as Lacticaseibacillus rhamnosus (previously L. rhamnosus) are associated with a reduction of atopic eczema, also known as dermatitis, due to anti-inflammatory cytokines secreted by this probiotic bacteria.[9] In addition, lactobacilli with other probiotic[37] organisms in ripened milk and yogurt aid development of immunity in the mucous intestine in humans by raising the number of LgA (+).
Oral health
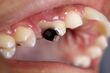
Some lactobacilli have been associated with cases of dental caries (cavities). Lactic acid can corrode teeth, and the Lactobacillus count in saliva has been used as a "caries test" for many years. Lactobacilli characteristically cause existing carious lesions to progress, especially those in coronal caries. The issue is, however, complex, as recent studies show probiotics can allow beneficial lactobacilli to populate sites on teeth, preventing streptococcal pathogens from taking hold and inducing dental decay. The scientific research of lactobacilli in relation to oral health is a new field and only a few studies and results have been published.[38][39] Some studies have provided evidence of certain Lactobacilli which can be a probiotic for oral health.[40] Some species, but not all, show evidence in defense to dental caries.[40] Due to these studies, there have been applications of incorporating such probiotics in chewing gum and lozenges.[40] There is also evidence of certain Lactobacilli that are beneficial in the defense of periodontal disease such as gingivitis and periodontitis.[40]
Food production
Lactobacilli comprise most food fermenting lactic acid bacteria [41][42] and are used as starter cultures in industry for controlled fermentation in the production of wine, yogurt, cheese, sauerkraut, pickles, beer, cider, kimchi, cocoa, kefir, and other fermented foods, as well as animal feeds and the bokashi soil amendment. Lactobacillus species are dominant in yogurt, cheese, and sourdough fermentations.[41][42] The antibacterial and antifungal activity of lactobacilli relies on production of bacteriocins and low molecular weight compounds that inhibits these microorganisms.[43][44]
Sourdough bread is made either spontaneously, by taking advantage of the bacteria naturally present in flour, or by using a "starter culture", which is a symbiotic culture of yeast and lactic acid bacteria growing in a water and flour medium.[45] The bacteria metabolize sugars into lactic acid, which lowers the pH of their environment and creates the signature sourness associated with yogurt, sauerkraut, etc.
In many traditional pickling processes, vegetables are submerged in brine, and salt-tolerant lactobacilli feed on natural sugars found in the vegetables. The resulting mix of salt and lactic acid is a hostile environment for other microbes, such as fungi, and the vegetables are thus preserved—remaining edible for long periods.[46]
Lactobacilli, especially pediococci and L. brevis, are some of the most common beer spoilage organisms. They are, however, essential to the production of sour beers such as Belgian lambics and American wild ales, giving the beer a distinct tart flavor.[47]
See also
- Lactobacillus L. anticaries
- Lactic acid fermentation
- MRS agar
- Pediococcus
- Probiotics
- Proteobiotics
- Carbon monoxide-releasing molecules
References
- ↑ 1.0 1.1 "Sur les ferments lactiques de l'industrie". Archives Néerlandaises des Sciences Exactes et Naturelles (Section 2) [Dutch Archives of Exact and Natural Sciences (Section 2)] 6: 212–243. 1901.
- ↑ "Comparative genomics of the lactic acid bacteria". Proceedings of the National Academy of Sciences of the United States of America 103 (42): 15611–6. October 2006. doi:10.1073/pnas.0607117103. PMID 17030793. Bibcode: 2006PNAS..10315611M.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Zheng, Jinshui; Wittouck, Stijn; Salvetti, Elisa; Franz, Charles M.A.P.; Harris, Hugh M.B.; Mattarelli, Paola; O’Toole, Paul W.; Pot, Bruno et al. (2020). "A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae". International Journal of Systematic and Evolutionary Microbiology 70 (4): 2782–2858. doi:10.1099/ijsem.0.004107. ISSN 1466-5026. PMID 32293557.
- ↑ Duar, Rebbeca M.; Lin, Xiaoxi B.; Zheng, Jinshui; Martino, Maria Elena; Grenier, Théodore; Pérez-Muñoz, María Elisa; Leulier, François; Gänzle, Michael et al. (August 2017). "Lifestyles in transition: evolution and natural history of the genus Lactobacillus". FEMS Microbiology Reviews 41 (Supp_1): S27–S48. doi:10.1093/femsre/fux030. ISSN 1574-6976. PMID 28673043.
- ↑ 5.0 5.1 "Vaginal microbiome: rethinking health and disease". Annual Review of Microbiology 66 (1): 371–89. 20 September 2012. doi:10.1146/annurev-micro-092611-150157. PMID 22746335.
- ↑ "Differences in vaginal microbiome in African American women versus women of European ancestry". Microbiology 160 (Pt 10): 2272–2282. October 2014. doi:10.1099/mic.0.081034-0. PMID 25073854.
- ↑ Lin, Xiaoxi B.; Wang, Tuo; Stothard, Paul; Corander, Jukka; Wang, Jun; Baines, John F.; Knowles, Sarah C. L.; Baltrūnaitė, Laima et al. (November 2018). "The evolution of ecological facilitation within mixed-species biofilms in the mouse gastrointestinal tract". The ISME Journal 12 (11): 2770–2784. doi:10.1038/s41396-018-0211-0. ISSN 1751-7370. PMID 30013162. Bibcode: 2018ISMEJ..12.2770L.
- ↑ "Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics". Microorganisms 4 (3): 35. September 2016. doi:10.3390/microorganisms4030035. PMID 27681929.
- ↑ 9.0 9.1 9.2 "Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease". Microbial Cell Factories 12 (71): 71. July 2013. doi:10.1186/1475-2859-12-71. PMID 23876056.
- ↑ Inglin R (2017). Combined Phenotypic-Genotypic Analyses of the Genus Lactobacillus and Selection of Cultures for Biopreservation of Fermented Food. ETHZ research collection (Ph.D. thesis). ETH Zurich. doi:10.3929/ethz-b-000214904. hdl:20.500.11850/214904.
- ↑ 11.0 11.1 11.2 11.3 Gänzle, Michael G (2015-04-01). "Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage" (in en). Current Opinion in Food Science. Food Microbiology • Functional Foods and Nutrition 2: 106–117. doi:10.1016/j.cofs.2015.03.001. ISSN 2214-7993. http://www.sciencedirect.com/science/article/pii/S2214799315000508.
- ↑ "Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria". Journal of Bacteriology 146 (3): 928–36. June 1981. doi:10.1128/JB.146.3.928-936.1981. PMID 6263860.
- ↑ Weinberg, E. D. (1997). "The Lactobacillus anomaly: total iron abstinence". Perspectives in Biology and Medicine 40 (4): 578–583. doi:10.1353/pbm.1997.0072. ISSN 0031-5982. PMID 9269745. https://pubmed.ncbi.nlm.nih.gov/9269745/.
- ↑ Li, Qing; Gänzle, Michael G. (December 2020). "Characterization of two extracellular arabinanases in Lactobacillus crispatus". Applied Microbiology and Biotechnology 104 (23): 10091–10103. doi:10.1007/s00253-020-10979-0. ISSN 1432-0614. PMID 33119797. https://pubmed.ncbi.nlm.nih.gov/33119797/.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med 8 (1): 46. April 2016. doi:10.1186/s13073-016-0296-x. PMID 27102537. "Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals".
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ↑ "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. March 2009. doi:10.1073/pnas.0812874106. PMID 19234110. "Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.".
IPA metabolism diagram - ↑ "3-Indolepropionic acid". University of Alberta. http://www.hmdb.ca/metabolites/HMDB02302. Retrieved 12 June 2018. "Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516) ... More recently it has been found that higher indole-3-propionic acid levels in serum/plasma are associated with reduced likelihood of type 2 diabetes and with higher levels of consumption of fiber-rich foods (PMID 28397877)
Origin: • Endogenous • Microbial" - ↑ "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. July 1999. doi:10.1074/jbc.274.31.21937. PMID 10419516. "[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.".
- ↑ "Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment". Journal of Bacteriology 196 (7): 1458–70. April 2014. doi:10.1128/JB.01439-13. PMID 24488312.
- ↑ "Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera" (in En). Nature Communications 6 (1): 8322. September 2015. doi:10.1038/ncomms9322. PMID 26415554. Bibcode: 2015NatCo...6.8322S.
- ↑ "Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina". Applied and Environmental Microbiology 82 (24): 7063–7073. December 2016. doi:10.1128/AEM.02385-16. PMID 27694231. Bibcode: 2016ApEnM..82.7063F.
- ↑ "Survey of compound microsatellites in multiple Lactobacillus genomes". Canadian Journal of Microbiology 61 (12): 898–902. December 2015. doi:10.1139/cjm-2015-0136. PMID 26445296.
- ↑ "Plasmids encode niche-specific traits in Lactobacillaceae". Microbial Genomics 7 (3). November 2020. doi:10.1099/mgen.0.000472. PMID 33166245.
- ↑ 24.0 24.1 "Lactobacillus". List of Prokaryotic names with Standing in Nomenclature (LPSN). https://lpsn.dsmz.de/genus/lactobacillus.
- ↑ "Lactobacillus" (in en). Bethesda, MD: National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=1578&lvl=3&keep=1&srchmode=1&unlock.
- ↑ Haakensen, Monique; Dobson, C. Melissa; Hill, Janet E.; Ziola, Barry (2009). "Reclassification of Pediococcus dextrinicus (Coster and White 1964) Back 1978 (Approved Lists 1980) as Lactobacillus dextrinicus comb. nov., and emended description of the genus Lactobacillus". International Journal of Systematic and Evolutionary Microbiology 59 (3): 615–621. doi:10.1099/ijs.0.65779-0. ISSN 1466-5026. PMID 19244449.
- ↑ 27.0 27.1 "Review article: fungal microbiota and digestive diseases". Alimentary Pharmacology & Therapeutics 39 (8): 751–66. April 2014. doi:10.1111/apt.12665. PMID 24612332. "In addition, GI fungal infection is reported even among those patients with normal immune status. Digestive system-related fungal infections may be induced by both commensal opportunistic fungi and exogenous pathogenic fungi. ...
In vitro, bacterial hydrogen peroxide or organic acids can inhibit C. albicans growth and virulence61
In vivo, Lactobacillus sp. can inhibit the GI colonisation and infection of C. albicans62
In vivo, C. albicans can suppress Lactobacillus sp. regeneration in the GI tract after antibiotic therapy63, 64". - ↑ 28.0 28.1 "Small intestinal fungal overgrowth". Current Gastroenterology Reports 17 (4): 16. April 2015. doi:10.1007/s11894-015-0436-2. PMID 25786900. "Small intestinal fungal overgrowth (SIFO) is characterized by the presence of excessive number of fungal organisms in the small intestine associated with gastrointestinal (GI) symptoms. Candidiasis is known to cause GI symptoms particularly in immunocompromised patients or those receiving steroids or antibiotics. However, only recently, there is emerging literature that an overgrowth of fungus in the small intestine of non-immunocompromised subjects may cause unexplained GI symptoms. ... Fungal-bacterial interaction may act in different ways and may either be synergistic or antagonistic or symbiotic [29]. Some bacteria such as Lactobacillus species can interact and inhibit both the virulence and growth of Candida species in the gut by producing hydrogen peroxide [30]. Any damage to the mucosal barrier or disruption of GI microbiota with chemotherapy or antibiotic use, inflammatory processes, activation of immune molecules and disruption of epithelial repair may all cause fungal overgrowth [27].".
- ↑ 29.0 29.1 29.2 29.3 "Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. albicans and attenuates the experimental candidiasis in Galleria mellonella". Virulence 6 (1): 29–39. February 2015. doi:10.4161/21505594.2014.981486. PMID 25654408.
- ↑ Axelsson, L. T.; Chung, T. C.; Dobrogosz, W. J.; Lindgren, S. E. (April 1988). "Production of a Broad Spectrum Antimicrobial Substance by Lactobacillus reuteri". Microbial Ecology in Health and Disease 2 (2): 131–136. doi:10.3109/08910608909140210.
- ↑ Brink, B. ten; Minekus, M.; van der Vossen, J.M.B.M.; Leer, R.J.; Huis in't Veld, J.H.J. (August 1994). "Antimicrobial activity of lactobacilli: preliminary characterization and optimization of production of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus M46". Journal of Applied Microbiology 77 (2): 140–148. doi:10.1111/j.1365-2672.1994.tb03057.x. PMID 7961186.
- ↑ 'Lactic Acid Bacteria and Their Uses in Animal Feeding to Improve Food Safety' in Advances in Food and Nutrition Research, Volume 50 (Elsevier),
- ↑ "Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis". The American Journal of Gastroenterology 109 (10): 1547–61; quiz 1546, 1562. October 2014. doi:10.1038/ajg.2014.202. PMID 25070051.
- ↑ 34.0 34.1 34.2 "Use of probiotics in the fight against Helicobacter pylori". World Journal of Gastrointestinal Pathophysiology 5 (4): 384–91. November 2014. doi:10.4291/wjgp.v5.i4.384. PMID 25400981.
- ↑ 35.0 35.1 35.2 Bernard, J.N.; Chinnaiyan, V.; Almeda, J.; Catala-Valentin, A.; Andl, C.D. Lactobacillus sp. Facilitate the Repair of DNA Damage Caused by Bile-Induced Reactive Oxygen Species in Experimental Models of Gastroesophageal Reflux Disease. Antioxidants 2023, 12, 1314. https://doi.org/10.3390/antiox12071314
- ↑ 36.0 36.1 "Vaginal microbiota and the use of probiotics". Interdisciplinary Perspectives on Infectious Diseases 2008: 256490. March 9, 2009. doi:10.1155/2008/256490. PMID 19343185.
- ↑ Ashraf, Shah, Rabia, Nagendra P (2014). "Immune system stimulation by probiotic microorganisms". Critical Reviews in Food Science and Nutrition 54 (7): 938–56. doi:10.1080/10408398.2011.619671. PMID 24499072.
- ↑ "Probiotics and oral health effects in children". International Journal of Paediatric Dentistry 18 (1): 3–10. January 2008. doi:10.1111/j.1365-263X.2007.00885.x. PMID 18086020.
- ↑ "Probiotics: contributions to oral health". Oral Diseases 13 (5): 443–51. September 2007. doi:10.1111/j.1601-0825.2007.01386.x. PMID 17714346.
- ↑ 40.0 40.1 40.2 40.3 Grenier, Daniel (October 2009). "Probiotics for Oral Health: Myth or Reality?". Professional Issues 75 (8): 585–590. PMID 19840501. https://www.cda-adc.ca/jcda/vol-75/issue-8/585.pdf.
- ↑ 41.0 41.1 Gänzle, Michael (2019), "Fermented Foods" (in en), Food Microbiology (John Wiley & Sons, Ltd): pp. 855–900, doi:10.1128/9781555819972.ch33, ISBN 978-1-68367-047-6, https://onlinelibrary.wiley.com/doi/abs/10.1128/9781555819972.ch33, retrieved 2020-11-28
- ↑ 42.0 42.1 Hutkins, Robert W., ed (2018) (in en). Microbiology and Technology of Fermented Foods, 2nd edition. Ames, Iowa, USA: Blackwell Publishing. doi:10.1002/9780470277515. ISBN 978-0-470-27751-5. http://doi.wiley.com/10.1002/9780470277515.
- ↑ "High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species". Journal of Microbiological Methods 114 (July 2015): 26–9. July 2015. doi:10.1016/j.mimet.2015.04.011. PMID 25937247.
- ↑ Inglin, Raffael (2017). PhD Thesis - Combined Phenotypic-Genotypic Analyses of the Genus Lactobacillus and Selection of Cultures for Biopreservation of Fermented Food (Doctoral Thesis). ETH Zurich. doi:10.3929/ethz-b-000214904. hdl:20.500.11850/214904.
- ↑ Gänzle, Michael G.; Zheng, Jinshui (2019-08-02). "Lifestyles of sourdough lactobacilli - Do they matter for microbial ecology and bread quality?". International Journal of Food Microbiology 302: 15–23. doi:10.1016/j.ijfoodmicro.2018.08.019. ISSN 1879-3460. PMID 30172443. https://pubmed.ncbi.nlm.nih.gov/30172443/.
- ↑ Priyanka, V; Ramesha, A; Gayathri, D; Vasudha, M (June 2021). "Molecular characterization of non-biogenic amines producing Lactobacillus plantarum GP11 isolated from traditional pickles using HRESI-MS analysis.". Journal of Food Science and Technology 58 (6): 2216–2226. doi:10.1007/s13197-020-04732-8. PMID 33967318.
- ↑ Carriglio, John; Budner, Drew; Thompson-Witrick, Katherine A. (November 2022). "Comparison Review of the Production, Microbiology, and Sensory Profile of Lambic and American Coolship Ales" (in en). Fermentation 8 (11): 646. doi:10.3390/fermentation8110646. ISSN 2311-5637.
External links
Wikidata ☰ Q1061596 entry
 |
