Chemistry:Examorelin
 | |
| Clinical data | |
|---|---|
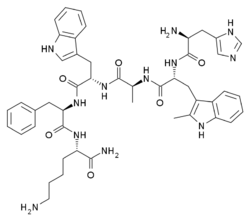
| Other names | L-Histidyl-2-methyl-D-tryptophyl-L-alanyl-L-tryptophyl-D-phenylalanyl-L-lysinamide |
| Routes of administration | Intravenous, subcutaneous, intranasal, oral[1] |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | ~55 minutes[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C47H58N12O6 |
| Molar mass | 887.059 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Examorelin (INN) (developmental code names EP-23905, MF-6003), also known as hexarelin, is a potent, synthetic, peptidic, orally-active, centrally-penetrant, and highly selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) and a growth hormone secretagogue which was developed by Mediolanum Farmaceutici.[3][4][5][6][7] It is a hexapeptide with the amino acid sequence His-D-2-methyl-Trp-Ala-Trp-D-Phe-Lys-NH2 which was derived from GHRP-6. These GH-releasing peptides have no sequence similarity to ghrelin, but mimic ghrelin by acting as agonists at the ghrelin receptor.[5][6]
Examorelin substantially and dose-dependently increases plasma levels of growth hormone (GH) in animals and humans.[2] In addition, similarly to pralmorelin (GHRP-2) and GHRP-6, it slightly and dose-dependently stimulates the release of prolactin, adrenocorticotropic hormone (ACTH), and cortisol in humans.[2][8] There are conflicting reports on the ability of examorelin to elevate insulin-like growth factor 1 (IGF-1) and insulin-like growth factor-binding protein 1 (IGFBP-1) levels in humans, with some studies finding no increase and others finding a slight yet statistically significant increase.[2][9][10][11] Examorelin does not affect plasma levels of glucose, luteinizing hormone (LH), follicle-stimulating hormone (FSH), or thyroid-stimulating hormone (TSH) in humans.[2]
Examorelin releases more GH than does growth hormone-releasing hormone (GHRH) in humans,[8][12] and produces synergistic effects on GH release in combination with GHRH, resulting in "massive" increases in plasma GH levels even with only low doses of examorelin.[13][14][15] Pre-administration of GH blunts the GH-releasing effect of examorelin, while, in contrast, fully abolishing the effect of GHRH.[14][16] Pre-treatment with IGF-1 also blunts the GH-elevating effect of examorelin.[17] Testosterone, testosterone enanthate, and ethinylestradiol, though not oxandrolone, have been found to significantly potentiate the GH-releasing effects of examorelin in humans.[18][19] In accordance, likely due to increases in sex steroid levels, puberty has also been found to significantly augment the GH-elevating actions of examorelin in humans.[20]
A partial and reversible tolerance to the GH-releasing effects of examorelin occurs in humans with long-term administration (50–75% decrease in efficacy over the course of weeks to months).[21][22]
Examorelin reached phase II clinical trials for the treatment of growth hormone deficiency and congestive heart failure but did not complete development and was never marketed.[6][23]
See also
- List of growth hormone secretagogues
References
- ↑ "Growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, after intravenous, subcutaneous, intranasal, and oral administration in man". The Journal of Clinical Endocrinology and Metabolism 78 (3): 693–698. March 1994. doi:10.1210/jcem.78.3.8126144. PMID 8126144.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Growth hormone-releasing activity of hexarelin in humans. A dose-response study". European Journal of Clinical Pharmacology 46 (5): 421–425. 1994. doi:10.1007/bf00191904. PMID 7957536.
- ↑ Dictionary of Pharmacological Agents. CRC Press. 21 November 1996. pp. 617–. ISBN 978-0-412-46630-4. https://books.google.com/books?id=Z_mfTTIApVEC&pg=PA617.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 117–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA117.
- ↑ 5.0 5.1 "Recent developments in ghrelin receptor ligands". ChemMedChem 2 (9): 1242–1259. September 2007. doi:10.1002/cmdc.200700015. PMID 17520591.
- ↑ 6.0 6.1 6.2 "Tesamorelin, a human growth hormone releasing factor analogue". Expert Opinion on Investigational Drugs 18 (3): 303–310. March 2009. doi:10.1517/13543780802707658. PMID 19243281.
- ↑ "Recent developments in ghrelin receptor (GHS-R1a) agonists and antagonists". Expert Opinion on Therapeutic Patents 12 (11): 1599–1618. 2002. doi:10.1517/13543776.12.11.1599.
- ↑ 8.0 8.1 "Effects of GHRP-2 and hexarelin, two synthetic GH-releasing peptides, on GH, prolactin, ACTH and cortisol levels in man. Comparison with the effects of GHRH, TRH and hCRH". Peptides 18 (6): 885–891. 1997. doi:10.1016/s0196-9781(97)00016-8. PMID 9285939.
- ↑ "Short-term administration of intranasal or oral Hexarelin, a synthetic hexapeptide, does not desensitize the growth hormone responsiveness in human aging". European Journal of Endocrinology 135 (4): 407–412. October 1996. doi:10.1530/eje.0.1350407. PMID 8921821.
- ↑ "Intranasal administration of the GHRP hexarelin accelerates growth in short children". Clinical Endocrinology 43 (5): 631–635. November 1995. doi:10.1111/j.1365-2265.1995.tb02929.x. PMID 8548949.
- ↑ "Short term effect of intranasal administration of hexarelin--a synthetic growth hormone-releasing peptide. Preliminary communication". Journal of Pediatric Endocrinology & Metabolism 8 (1): 43–45. 1995. doi:10.1515/jpem.1995.8.1.43. PMID 7584696.
- ↑ "Metabolic modulation of the growth hormone-releasing activity of hexarelin in man". Metabolism 44 (1): 134–138. January 1995. doi:10.1016/0026-0495(95)90300-3. PMID 7854159.
- ↑ "Hexarelin-induced growth hormone, cortisol, and prolactin release: a dose-response study". The Journal of Clinical Endocrinology and Metabolism 81 (12): 4338–4341. December 1996. doi:10.1210/jcem.81.12.8954038. PMID 8954038.
- ↑ 14.0 14.1 "Mechanisms underlying the negative growth hormone (GH) autofeedback on the GH-releasing effect of hexarelin in man". Metabolism 46 (1): 83–88. January 1997. doi:10.1016/s0026-0495(97)90173-6. PMID 9005975.
- ↑ "Modulation of growth hormone-releasing activity of hexarelin in man". Neuroendocrinology 61 (1): 51–56. January 1995. doi:10.1159/000126827. PMID 7731498.
- ↑ "Hexarelin induced growth hormone release is influenced by exogenous growth hormone". Clinical Endocrinology 43 (5): 617–621. November 1995. doi:10.1111/j.1365-2265.1995.tb02927.x. PMID 8548947.
- ↑ "Anabolic Dysfunction". Bioactive Peptides: Applications for Improving Nutrition and Health. CRC Press. 23 June 2010. pp. 292–. ISBN 978-1-4398-1363-8. https://books.google.com/books?id=JJ_MBQAAQBAJ&pg=PA292.
- ↑ "The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids". The Journal of Clinical Endocrinology and Metabolism 82 (3): 861–864. March 1997. doi:10.1210/jcem.82.3.3795. PMID 9062497.
- ↑ "The growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, in short normal and obese children and in hypopituitary subjects". The Journal of Clinical Endocrinology and Metabolism 80 (2): 674–678. February 1995. doi:10.1210/jcem.80.2.7852535. PMID 7852535.
- ↑ "Growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, before and during puberty". The Journal of Clinical Endocrinology and Metabolism 80 (4): 1090–1094. April 1995. doi:10.1210/jcem.80.4.7714074. PMID 7714074.
- ↑ "Growth hormone status during long-term hexarelin therapy". The Journal of Clinical Endocrinology and Metabolism 83 (5): 1644–1649. May 1998. doi:10.1210/jcem.83.5.4812. PMID 9589671.
- ↑ Growth Hormone Secretagogues: Basic Findings and Clinical Implications. Elsevier. 1999. pp. 178–. ISBN 978-0-444-82933-7. https://books.google.com/books?id=pDabYbzcGSQC&pg=PA178.
- ↑ "Discontinued drugs in 2005: cardiovascular drugs". Expert Opinion on Investigational Drugs 15 (11): 1299–1308. November 2006. doi:10.1517/13543784.15.11.1299. PMID 17040192.
 |

