Chemistry:Protactinium(IV) bromide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Protactinium(IV) tetrabromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| PaBr4 | |
| Molar mass | 550.652 |
| Appearance | red crystals |
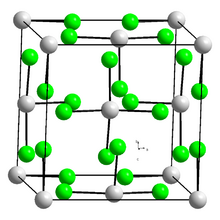
| Structure | |
| tetragonal crystal system[1][2],tI20 | |
| I41/amd , No. 141 | |
| Related compounds | |
Other anions
|
Protactinium(IV) fluoride Protactinium(IV) chloride Protactinium(IV) iodide |
Other cations
|
Uranium(IV) bromide Thorium(IV) bromide Praseodymium(III) bromide |
Related compounds
|
Protactinium(V) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Protactinium(IV) bromide is an inorganic compound. It is an actinide halide, composed of protactinium and bromine. It is radioactive, and has the chemical formula of PaBr4. It may be due to the brown color of bromine that causes the appearance of protactinium(IV) bromide to be brown crystals. Its crystal structure is tetragonal.[1][2] Protactinium(IV) bromide is sublimed in a vacuum at 400 °C.[1] The protactinium(IV) halide closest in structure to protactinium(IV) bromide is protactinium(IV) chloride.
Preparation
Protactinium(IV) bromide can be prepared by reacting protactinium(V) bromide with hydrogen gas or aluminium:[3]
- [math]\displaystyle{ \mathsf{ 3 PaBr_5 + Al \ \xrightarrow{400-450^oC}\ 3 PaBr_4 + AlBr_3 } }[/math]
- [math]\displaystyle{ \mathsf{ 2 PaBr_5 + H_2 \ \xrightarrow{800^oC}\ 2 PaBr_4 + 2 HBr } }[/math]
Properties
Protactinium(IV) bromide reacts with antimony trioxide to form protactinium bromate:[4]
- [math]\displaystyle{ \mathsf{ 3 PaBr_4 + Sb_2O_3 \ \xrightarrow{150-200^oC}\ 3 PaOBr_2 + 2 SbBr_3 } }[/math]
See also
References
- ↑ 1.0 1.1 1.2 Tahri, Y; Chermette, H; Elkhatib, N; Krupa, J; Simoni, E (1990). "Electronic structures of thorium and protactinium halide clusters of [ThX8]4− type". Journal of the Less Common Metals 158: 105–116. doi:10.1016/0022-5088(90)90436-N.
- ↑ 2.0 2.1 Brown, D.; Petcher, T. J.; Smith, A. J. (1968). "Crystal Structures of some Protactinium Bromides". Nature 217 (5130): 737. doi:10.1038/217737a0. Bibcode: 1968Natur.217..737B.
- ↑ Руководство по неорганическому синтезу: В 6-ти т.. 4. М.: Мир. 1985.
- ↑ Morss, Lester R.; Edelstein, Norman M.; Fuger, J. (2010). The chemistry of the actinide and transactinide elements. Volumes 1-6. Dordrecht: Springer. p. 874. ISBN 978-94-007-0211-0. OCLC 682907473.
 |

