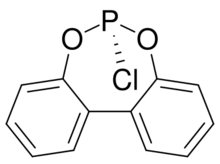
Chemistry:2,2'-Biphenylene phosphorochloridite
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-Chloro-6H-dibenzo[d,f][1,3,2]dioxaphosphepine | |
| Other names
1,1′-Biphenyl-2,2′-diyl phosphorochloridite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H8ClO0P | |
| Molar mass | 218.62 g·mol−1 |
| Appearance | white solid |
| Melting point | 63 °C (145 °F; 336 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,2′-Biphenylene phosphorochloridite is the name for a polycyclic organophosphorus compound with the formula C12H8O2PCl. It is a precursor to diphosphite ligands such as BiPhePhos by reaction with suitable diols. 2,2′-Biphenylene phosphorochloridites, which is a white solid, is prepared from 2,2′-biphenol and phosphorus trichloride. It is prepared by the reaction of 2,2′-biphenol and phosphorus trichloride.[1][2]
References
- ↑ Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-Olefins". Journal of the American Chemical Society 115 (5): 2066–2068. doi:10.1021/ja00058a079.
- ↑ Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics 15 (2): 835–847. doi:10.1021/OM950549K. http://dare.uva.nl/personal/pure/en/publications/bulky-diphosphite-modified-rhodium-catalyst-hydroformylation-and-characterization(c06c2654-cecb-4e97-84ba-f1fdfb51ad35).html.
 |

