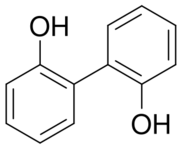
Chemistry:2,2'-Biphenol
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
[1,1′-Biphenyl]-2,2′-diol | |
| Other names
o,o′-Dihydroxybiphenyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1638363 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 51261 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H10O2 | |
| Molar mass | 186.210 g·mol−1 |
| Appearance | white solid |
| Melting point | 109 °C (228 °F; 382 K) |
| Boiling point | 320 °C (608 °F; 593 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H315, H318, H319, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2,2′-Biphenol is an organic compound with the formula (C6H4OH)2. It is one of three symmetrical isomers of biphenol. A white solid, it is a precursor to diphosphite ligands that are used to support industrial hydroformylation catalysis.[1][2] thumb|244px|left|BiPhePhos is representative diphosphite ligand derived from 2,2′-biphenol.
Synthesis
The chemical can be made by a hydrolysis reaction that opens the central ring of dibenzofuran. Alternatively, it can be produced from 2,4-di-tert-butylphenol in two steps. The first step entails oxidative coupling to give the 2,2′-biphenol with four tert-butyl substituents. This species then undergoes debutylation.[3]
See also
References
- ↑ Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-Olefins". Journal of the American Chemical Society 115 (5): 2066–2068. doi:10.1021/ja00058a079.
- ↑ Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics 15 (2): 835–847. doi:10.1021/OM950549K. http://dare.uva.nl/personal/pure/en/publications/bulky-diphosphite-modified-rhodium-catalyst-hydroformylation-and-characterization(c06c2654-cecb-4e97-84ba-f1fdfb51ad35).html.
- ↑ Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J. et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
 |

