Chemistry:4,4'-Bipyridine
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-Bipyridine | |
| Identifiers | |
3D model (JSmol)
|
|
| 113176 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 3759 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H8N2 | |
| Molar mass | 156.188 g·mol−1 |
| Melting point | 114 °C (237 °F; 387 K) |
| Boiling point | 305 °C (581 °F; 578 K) |
| Structure | |
| 0 D | |
| Related compounds | |
Related compounds
|
2,2′-Bipyridine Pyridine 4-Pyridylnicotinamide Terpyridine Biphenyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
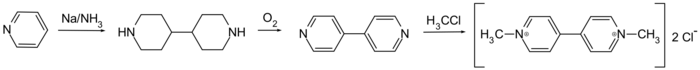
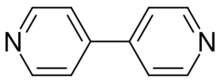
4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is an organic compound with the formula (C
5H
4N)
2. It is one of several isomers of bipyridine. It is a colorless solid that is soluble in organic solvents. is mainly used as a precursor to N,N′-dimethyl-4,4′-bipyridinium [(C5H4NCH3)2]2+, known as paraquat.
History
4,4′-Bipyridine was first obtained in 1868 by the Scottish chemist Thomas Anderson via heating pyridine with sodium metal.[1] However, Anderson's empirical formula for 4,4′-bipyridine was incorrect.[2] The correct empirical formula, and the correct molecular structure, for 4,4′-bipyridine was provided in 1882 by the Austrian chemist Hugo Weidel and his student M. Russo.[3]
Uses
4,4'-Bipyridine is an intermediate in the production of paraquat, a widely-used herbicide. In this process, pyridine is oxidized to 4,4'-bipyridine in a coupling reaction, followed by dimethylation to form paraquat.[4]
Reactions
The reducing agent is N,N'-bis(trimethylsilyl)-4,4'-bipyridinylidene is produced by reduction of 4,4'-bipyridine in the presence of trimethylsilyl chloride (Me = CH3):
- NC
5H
4C
5H
4N + 2 Li + 2 Me
3SiCl → Me
3SiNC
5H
4C
5H
4NSiMe
3 + 2 LiCl
The silylated derivative, which is red, is used in salt-free reductions.[5]
4,4′-bipyridine forms a variety of coordination polymers.[6]
References
- ↑ See:
- Anderson, Thomas (1868). "On the products of the destructive distillation of animal substances. Part V.". Transactions of the Royal Society of Edinburgh 25: 205–216. doi:10.1017/S0080456800028167. https://babel.hathitrust.org/cgi/pt?id=mdp.39015032051941&view=1up&seq=249. Anderson called 4,4′-bipyridine "Dipyridine".
- German translation: Anderson, Th. (1870). "Ueber die Producte der trockenen Destillation thierischer Materien. Fünfter Theil." (in de). Annalen der Chemie und Pharmacie 154: 270–286. doi:10.1002/jlac.18701540303. https://babel.hathitrust.org/cgi/pt?id=uc1.c036497839&view=1up&seq=280.
- See also: Fehling, Hermann Christian von, ed (1890) (in de). Neues Handwörterbuch der Chemie. 5. Braunschweig, Germany: Friedrich Vieweg und Sohn. p. 974. https://books.google.com/books?id=ZvME1LB2GpMC&pg=PA974. See γ-Dipyridyl.
- ↑ Anderson gave the empirical formula for 4,4′-bipyridine as C10H10N2. See:
- (Anderson, 1868), p. 209.
- (Fehling, 1890), p. 974 (γ-Dipyridyl).
- ↑ Weidel, H.; Russo, M. (1882). "Studien über das Pyridin" (in de). Monatshefte für Chemie 3: 850–885. doi:10.1007/BF01516855. https://babel.hathitrust.org/cgi/pt?id=uiug.30112025861714&view=1up&seq=866. The empirical formula for 4,4′-bipyridine (γ-Dipyridyl) appears on p. 856 ; the molecular structure of 4,4′-bipyridine (γ-Dipyridyl) appears on p. 867.
- ↑ "Paraquat and Diquat". IPCS INCHEM. http://www.inchem.org/documents/ehc/ehc/ehc39.htm.
- ↑ Tsurugi, Hayato; Mashima, Kazushi (2019). "Salt-Free Reduction of Transition Metal Complexes by Bis(trimethylsilyl)cyclohexadiene, -dihydropyrazine, and -4,4′-bipyridinylidene Derivatives". Accounts of Chemical Research 52 (3): 769–779. doi:10.1021/acs.accounts.8b00638. PMID 30794373.
- ↑ Biradha, K.; Sarkar, M.; Rajput, L. (2006). "Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms". Chemical Communications (40): 4169–79. doi:10.1039/B606184B. PMID 17031423.
 |