Chemistry:Iminium
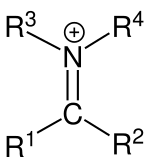
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1
R2
C=NR3
R4
]+
.[1] They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries: the central C=N unit is nearly coplanar with all four substituents. Unsymmetrical iminium cations can exist as cis and trans isomers. The C=N bonds, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. The C=N distance is slightly shorter in iminium cations than in the parent imine, and computational studies indicate that the C=N bonding is also stronger in iminium vs imine, although the C=N distance contracts only slightly. These results indicate that the barrier for rotation is higher than in the parent imines.[3][4]
Formation
Iminium cations are obtained by protonation and alkylation of imines:
- R'N=CR
2 + H+
→ [R'NH=CR
2]+ - R'N=CR
2 + R+
→ [R'RN=CR
2]+
They also are generated by the condensation of secondary amines with ketones or aldehydes:
- O=CR
2 + R'
2NH + H+
⇌ [R'
2N=CR
2]+
+ H
2O
This rapid, reversible reaction is one step in "iminium catalysis".[5]
More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine.[6]
Occurrence
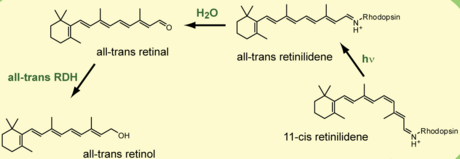
Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are encountered in synthetic organic chemistry. thumb|right|"[[Eschenmoser's salt" is a well known example of an iminium salt.[7]]]
Reactions
Iminium salts hydrolyse to give the corresponding ketone or aldehyde:[8]
- [R
2N=CR
2]+
+ H
2O → [R
2NH
2]+
+ O=CR
2
Iminium cations are reduced to the amines, e.g. by sodium cyanoborohydride. Iminium cations are intermediates in the reductive amination of ketones and aldehydes.
Unsymmetrical iminium cations undergo cis-trans isomerization. The isomerization is catalyzed by nucleophiles, which add to the unsaturated carbon, breaking the C=N double bond.[3]
Named reactions involving iminium species
- Aza-Cope rearrangement[9]
- Beckmann rearrangement
- Duff reaction
- Mannich reaction
- Pictet-Spengler reaction
- Stephen aldehyde synthesis
- Stork enamine alkylation
- Vilsmeier-Haack reaction and Vilsmeier reagent
Iminylium ions
Iminylium ions have the general structure R2C=N+. They form a subclass of nitrenium ions.[10]
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "iminium compounds". doi:10.1351/goldbook.I02958
- ↑ Guido M. Böttger Roland Fröhlich Ernst‐Ulrich Würthwein (2000). "Electrophilic Reactivity of a 2‐Azaallenium and of a 2‐Azaallylium Ion". European Journal of Organic Chemistry 2000 (8): 1589–1593. doi:10.1002/(SICI)1099-0690(200004)2000:8<1589::AID-EJOC1589>3.0.CO;2-T..
- ↑ 3.0 3.1 Johnson, James E.; Morales, Nora M.; Gorczyca, Andrea M.; Dolliver, Debra D.; McAllister, Michael A. (2001). "Mechanisms of Acid-Catalyzed Z/E Isomerization of Imines". The Journal of Organic Chemistry 66 (24): 7979–7985. doi:10.1021/jo010067k. PMID 11722194.
- ↑ Wang, Youliang; Poirier, Raymond A. (1997). "Factors That Influence the CN Stretching Frequency in Imines". The Journal of Physical Chemistry A 101 (5): 907–912. doi:10.1021/jp9617332. Bibcode: 1997JPCA..101..907W.
- ↑ Erkkilä, Anniinä; Majander, Inkeri; Pihko, Petri M. (2007). "Iminium Catalysis". Chem. Rev. 107 (12): 5416–70. doi:10.1021/cr068388p. PMID 18072802.
- ↑ Hafner, Klaus; Meinhardt, Klaus-Peter (1984). "Azulene". Organic Syntheses 62: 134. doi:10.15227/orgsyn.062.0134.
- ↑ E. F. Kleinman (2004). "Dimethylmethyleneammonium Iodide and Chloride". Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rd346.
- ↑ C. Schmit; J. B. Falmagne; J. Escudero; H. Vanlierde; L. Ghosez (1990). "A General Synthesis of Cyclobutanones from Olefins and Tertiary Amides: 3-Hexylcyclobutanone". Org. Synth. 69: 199. doi:10.15227/orgsyn.069.0199.
- ↑ Grieco, P. A.; Larsen, S. D. (1990). "Iminium Ion-Based Diels–Alder Reactions: N-Benzyl-2-Azanorborene". Organic Syntheses 68: 206. doi:10.15227/orgsyn.068.0206. http://www.orgsyn.org/Content/pdfs/procedures/CV8P0031.pdf.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "iminylium ions". doi:10.1351/goldbook.I02964
 |