Biology:Rhodopsin
 Generic protein structure example |
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene[1] and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction in rods. Rhodopsin mediates dim light vision and thus is extremely sensitive to light.[2] When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which the rods are more sensitive.[3] Defects in the rhodopsin gene cause eye diseases such as retinitis pigmentosa and congenital stationary night blindness.
Names
Rhodopsin was discovered by Franz Christian Boll in 1876.[4][5][6] The name rhodospsin derives from Ancient Greek ῥόδον (rhódon) for "rose", due to its pinkish color, and ὄψις (ópsis) for "sight".[7] It was coined in 1878 by the German physiologist Wilhelm Friedrich Kühne (1837-1900).[8][9]
When George Wald discovered that rhodopsin is a holoprotein, consisting of retinal and an apoprotein, he called it opsin, which today would be described more narrowly as apo-rhodopsin.[10] Today, the term opsin refers more broadly to the class of G-protein-coupled receptors that bind retinal and as a result become a light sensitive photoreceptor, including all closely related proteins.[11][12][13][lower-alpha 1] When Wald and colleges later isolated iodopsin from chicken retinas, thereby discovering the first known cone opsin, they called apo-iodopsin photopsin (for its relation to photopic vision) and apo-rhodopsin scotopsin (for its use in scotopic vision).[14]
General
Rhodopsin is a protein found in the outer segment discs of rod cells. It mediates scotopic vision, which is monochromatic vision in dim light.[3][15] Rhodopsin most strongly absorbs green-blue light (~500 nm)[16][17] and appears therefore reddish-purple, hence the archaic term "visual purple".
Several closely related opsins differ only in a few amino acids and in the wavelengths of light that they absorb most strongly. Humans have, including rhodopsin, nine opsins,[11] as well as cryptochrome (light-sensitive, but not an opsin).[18]
Structure

Rhodopsin, like other opsins, is a G-protein-coupled receptor (GPCR).[19][20] GPCRs are chemoreceptors that embed in the lipid bilayer of the cell membranes and have seven transmembrane domains forming a binding pocket for a ligand.[21][22] The ligand for rhodopsin is the vitamin A-based chromophore 11-cis-retinal,[23][24][25][26][27] which lies horizontally to the cell membrane[28] and is covalently bound to a lysine residue (lys296)[29] in the seventh transmembrane domain[30][28] through a Schiff-base.[31][32] However, 11-cis-retinal only blocks the binding pocket and does not activate rhodopsin. It is only activated when 11-cis-retinal absorbs a photon of light and isomerizes to all-trans-retinal,[33][34] the receptor activating form,[35][36] causing conformal changes in rhodopsin (bleaching),[35] which activate a phototransduction cascade.[37] Thus, a chemoreceptor is converted to a light or photo(n)receptor.[12]
The retinal binding lysine is conserved in almost all opsins, only a few opsins having lost it during evolution.[12] Opsins without the lysine are not light sensitive,[38][39][40] including rhodopsin. Rhodopsin is made constitutively (continuously) active by some of those mutations even without light.[41][42][43] Also wild-type rhodopsin is constitutively active, if no 11-cis-retinal is bound, but much less.[44] Therefore 11-cis-retinal is an inverse agonist. Such mutations are one cause of autosomal dominant retinitis pigmentosa.[43] Artificially, the retinal binding lysine can be shifted to other positions, even into other transmembrane domains, without changing the activity.[45]
The rhodopsin of cattle has 348 amino acids, the retinal binding lysine being Lys296. It was the first opsin whose amino acid sequence[46] and 3D-structure were determined.[28] Its structure has been studied in detail by x-ray crystallography on rhodopsin crystals.[47] Several models (e.g., the bicycle-pedal mechanism, hula-twist mechanism) attempt to explain how the retinal group can change its conformation without clashing with the enveloping rhodopsin protein pocket.[48][49][50] Recent data support that rhodopsin is a functional monomer, instead of a dimer, which was the paradigm of G-protein-coupled receptors for many years.[51]
Within its native membrane, rhodopsin is found at a high density facilitating its ability to capture photons. Due to its dense packing within the membrane, there is a higher chance of rhodopsin capturing proteins. However, the high density also provides a disadvantage when it comes to G protein signaling because the diffusion becomes more difficult in a crowded membrane that is packed with the receptor, rhodopsin.[52]
Phototransduction
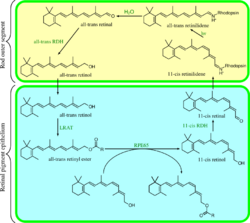
Rhodopsin is an essential G-protein coupled receptor in phototransduction.
Activation
In rhodopsin, the aldehyde group of retinal is covalently linked to the amino group of a lysine residue on the protein in a protonated Schiff base (-NH+=CH-).[29] When rhodopsin absorbs light, its retinal cofactor isomerizes from the 11-cis to the all-trans configuration, and the protein subsequently undergoes a series of relaxations to accommodate the altered shape of the isomerized cofactor. The intermediates formed during this process were first investigated in the laboratory of George Wald, who received the Nobel prize for this research in 1967.[53] The photoisomerization dynamics has been subsequently investigated with time-resolved IR spectroscopy and UV/Vis spectroscopy. A first photoproduct called photorhodopsin forms within 200 femtoseconds after irradiation, followed within picoseconds by a second one called bathorhodopsin with distorted all-trans bonds. This intermediate can be trapped and studied at cryogenic temperatures, and was initially referred to as prelumirhodopsin.[54] In subsequent intermediates lumirhodopsin and metarhodopsin I, the Schiff's base linkage to all-trans retinal remains protonated, and the protein retains its reddish color. The critical change that initiates the neuronal excitation involves the conversion of metarhodopsin I to metarhodopsin II, which is associated with deprotonation of the Schiff's base and change in color from red to yellow.[55]
Phototransduction cascade
The product of light activation, Metarhodopsin II, initiates the visual phototransduction second messenger pathway by stimulating the G-protein transducin (Gt), resulting in the liberation of its α subunit. This GTP-bound subunit in turn activates a cGMP phosphodiesterase. The cGMP phosphodiesterase hydrolyzes (breaks down) cGMP, lowering its local concentration so it can no longer activate cGMP-dependent cation channels. This leads to the hyperpolarization of photoreceptor cells, changing the rate at which they release transmitters.[56][37]
Deactivation
Meta II (metarhodopsin II) is deactivated rapidly after activating transducin by rhodopsin kinase and arrestin.[57] Rhodopsin pigment must be regenerated for further phototransduction to occur. This means replacing all-trans-retinal with 11-cis-retinal and the decay of Meta II is crucial in this process. During the decay of Meta II, the Schiff base link that normally holds all-trans-retinal and the apoprotein opsin (aporhodopsin) is hydrolyzed and becomes Meta III. In the rod outer segment, Meta III decays into separate all-trans-retinal and opsin.[57] A second product of Meta II decay is an all-trans-retinal opsin complex in which the all-trans-retinal has been translocated to second binding sites. Whether the Meta II decay runs into Meta III or the all-trans-retinal opsin complex seems to depend on the pH of the reaction. Higher pH tends to drive the decay reaction towards Meta III.[57]
Diseases of the retina
Mutations in the rhodopsin gene contribute majorly to various diseases of the retina such as retinitis pigmentosa. In general, the defect rhodopsin aggregates with ubiquitin in inclusion bodies, disrupts the intermediate filament network, and impairs the ability of the cell to degrade non-functioning proteins, which leads to photoreceptor apoptosis.[58] Other mutations on rhodopsin lead to X-linked congenital stationary night blindness, mainly due to constitutive activation, when the mutations occur around the chromophore binding pocket of rhodopsin.[59] Several other pathological states relating to rhodopsin have been discovered including poor post-Golgi trafficking, dysregulative activation, rod outer segment instability and arrestin binding.[59]
See also
- Bacteriorhodopsin, used in some halobacteria as a light-driven proton pump.
Explanatory notes
References
- ↑ "RHO rhodopsin [Homo sapiens (human)"]. https://www.ncbi.nlm.nih.gov/gene/6010.
- ↑ "Rhodopsin structure and function". Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Biomembranes: A Multi-Volume Treatise. 2. Greenwich, Conn: JAI Press. 1996. pp. 1–32. doi:10.1016/S1874-5342(07)80004-3. ISBN 978-1-55938-659-3.
- ↑ 3.0 3.1 "Characterization of the primary photochemical events in bacteriorhodopsin and rhodopsin". Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Biomembranes: A Multi-Volume Treatise. 2. Greenwich, Conn: JAI Press. 1996. pp. 33–140. doi:10.1016/S1874-5342(07)80005-5. ISBN 978-1-55938-659-3.
- ↑ Encyclopedia of the Neurological Sciences. Academic Press. 29 April 2014. pp. 441–. ISBN 978-0-12-385158-1. https://books.google.com/books?id=hfjSVIWViRUC&pg=PA441.
- ↑ Photophysiology: General Principles; Action of Light on Plants. Elsevier. 24 September 2013. p. 9. ISBN 978-1-4832-6227-7. https://books.google.com/books?id=16zSBAAAQBAJ&pg=PA9. Retrieved 23 September 2015.
- ↑ "Zur Anatomie und Physiologie der Retina" (in German). Archiv für Anatomie und Physiologie, Physiologische Abtheilung: 4–35. 1877. https://books.google.com/books?id=JFwoAQAAIAAJ&pg=PA4.
- ↑ "Rhodopsin: History and Etymology for rhodopsin". https://www.merriam-webster.com/dictionary/rhodopsin.
- ↑ See:
- Merriam-Webster Online Dictionary: Rhodopsin: History and Etymology for rhodopsin
- "Untersuchungen über den Sehpurpur" (in German). Untersuchungen aus dem Physiologischen Institute der Universität Heidelberg 1: 139–218. 1878. https://www.biodiversitylibrary.org/item/97176#page/189/mode/1up. From p. 181: "Was den Sehpurpur im Dunkel ändert, pflegt es z. Th. [= zum Theil] in derselben Weise zu thun, wie das Licht, d.h. erst eine gelbe Materie, dann farblose Substanz hervorzubringen. Der Kürze wegen und um dem Auslande unsere Bezeichnungen zugänglich zu machen, kann man sagen, Rhodopsin werde erst in Xanthopsin, dieses in Leukopsin zersetzt." (That which alters visual purple in the dark usually acts to some extent in the same way as light, that is, first producing a yellow material, then a colorless substance. For the sake of brevity, and in order to make our designations more accessible to foreigners, we can say that rhodopsin is first degraded into xanthopsin [- visual yellow], and [then] this is degraded into leucopsin [- visual white].)
- ↑ "Visual purple (sehpurpur)". Perception 37 (11): 1617–1620. November 2008. doi:10.1068/p3711ed. PMID 19189727.
- ↑ "The photochemical basis of rod vision". Journal of the Optical Society of America 41 (12): 949–956. December 1951. doi:10.1364/josa.41.000949. PMID 14908734.
- ↑ 11.0 11.1 "The opsins". Genome Biology 6 (3): 213. 2005. doi:10.1186/gb-2005-6-3-213. PMID 15774036.
- ↑ 12.0 12.1 12.2 "The Gluopsins: Opsins without the Retinal Binding Lysine". Cells 11 (15): 2441. August 2022. doi:10.3390/cells11152441. PMID 35954284.
 Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 13.0 13.1 "Rhodopsin, light-sensor of vision". Progress in Retinal and Eye Research 93: 101116. March 2023. doi:10.1016/j.preteyeres.2022.101116. PMID 36273969.
- ↑ "Iodopsin". The Journal of General Physiology 38 (5): 623–681. May 1955. doi:10.1085/jgp.38.5.623. PMID 14367777.
- ↑ "Rhodopsin". Britannica.com. http://www.britannica.com/science/rhodopsin.
- ↑ "Human rhodopsin". Science 127 (3292): 222–226. January 1958. doi:10.1126/science.127.3292.222. PMID 13495499. Bibcode: 1958Sci...127..222W.
- ↑ "Visual pigments of rods and cones in a human retina". The Journal of Physiology 298 (1): 501–511. January 1980. doi:10.1113/jphysiol.1980.sp013097. PMID 7359434.
- ↑ "Human cryptochrome exhibits light-dependent magnetosensitivity". Nature Communications 2: 356. June 2011. doi:10.1038/ncomms1364. PMID 21694704. Bibcode: 2011NatCo...2..356F.
- ↑ "G protein involvement in receptor-effector coupling". The Journal of Biological Chemistry 263 (6): 2577–2580. February 1988. doi:10.1016/s0021-9258(18)69103-3. PMID 2830256.
- ↑ "Fingerprinting G-protein-coupled receptors". Protein Engineering 7 (2): 195–203. February 1994. doi:10.1093/protein/7.2.195. PMID 8170923.
- ↑ "Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin". Nature 321 (6065): 75–79. May 1986. doi:10.1038/321075a0. PMID 3010132. Bibcode: 1986Natur.321...75D.
- ↑ "Ligand binding to the beta-adrenergic receptor involves its rhodopsin-like core". Nature 326 (6108): 73–77. March 1987. doi:10.1038/326073a0. PMID 2881211. Bibcode: 1987Natur.326...73D.
- ↑ "Carotenoids and the Vitamin A Cycle in Vision". Nature 134 (3376): 65. July 1934. doi:10.1038/134065a0. Bibcode: 1934Natur.134...65W.
- ↑ "Hindered Cis Isomers of Vitamin a and Retinene: The Structure of the Neo-B Isomer". Proceedings of the National Academy of Sciences of the United States of America 41 (7): 438–451. July 1955. doi:10.1073/pnas.41.7.438. PMID 16589696. Bibcode: 1955PNAS...41..438W.
- ↑ "The neo-b isomer of vitamin A and retinene". The Journal of Biological Chemistry 222 (2): 865–877. October 1956. doi:10.1016/S0021-9258(20)89944-X. PMID 13367054.
- ↑ "The Synthesis and Configuration of Neo-B Vitamin A and Neoretinine b". Journal of the American Chemical Society 78 (11): 2651–2652. June 1956. doi:10.1021/ja01592a095.
- ↑ "HINDERED CIS ISOMERS OF VITAMIN A AND RETINENE: THE STRUCTURE OF THE NEO-b ISOMER". Proceedings of the National Academy of Sciences of the United States of America 42 (9): 578–580. September 1956. doi:10.1073/pnas.42.9.578. PMID 16589909. Bibcode: 1956PNAS...42..578O.
- ↑ 28.0 28.1 28.2 "Crystal structure of rhodopsin: A G protein-coupled receptor". Science 289 (5480): 739–745. August 2000. doi:10.1126/science.289.5480.739. PMID 10926528. Bibcode: 2000Sci...289..739P.
- ↑ 29.0 29.1 "Site of attachment of retinal in rhodopsin". Nature 216 (5121): 1178–1181. December 1967. doi:10.1038/2161178a0. PMID 4294735. Bibcode: 1967Natur.216.1178B.
- ↑ "The structure of bovine rhodopsin". Biophysics of Structure and Mechanism 9 (4): 235–244. 1983. doi:10.1007/BF00535659. PMID 6342691.
- ↑ "Rhodopsin and indicator yellow". Nature 171 (4350): 469–471. March 1953. doi:10.1038/171469a0. PMID 13046517. Bibcode: 1953Natur.171..469C.
- ↑ "Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue". The Biochemical Journal 59 (1): 122–128. January 1955. doi:10.1042/bj0590122. PMID 14351151.
- ↑ "The Action of Light on Rhodopsin". Proceedings of the National Academy of Sciences of the United States of America 44 (2): 130–139. February 1958. doi:10.1073/pnas.44.2.130. PMID 16590155. Bibcode: 1958PNAS...44..130H.
- ↑ "The mechanism of bleaching rhodopsin". Annals of the New York Academy of Sciences 74 (2): 266–280. November 1959. doi:10.1111/j.1749-6632.1958.tb39550.x. PMID 13627857. Bibcode: 1959NYASA..74..266K.
- ↑ 35.0 35.1 "Crystal structure of metarhodopsin II". Nature 471 (7340): 651–655. March 2011. doi:10.1038/nature09789. PMID 21389988. Bibcode: 2011Natur.471..651C.
- ↑ "Molecular basis of visual excitation". Science 162 (3850): 230–239. October 1968. doi:10.1126/science.162.3850.230. PMID 4877437. Bibcode: 1968Sci...162..230W.
- ↑ 37.0 37.1 "Evolution and diversity of opsins". Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1 (1): 104–111. January 2012. doi:10.1002/wmts.6.
- ↑ "Chromophore-Independent Roles of Opsin Apoproteins in Drosophila Mechanoreceptors". Current Biology 29 (17): 2961–2969.e4. September 2019. doi:10.1016/j.cub.2019.07.036. PMID 31447373.
- ↑ "Functions of Opsins in Drosophila Taste". Current Biology 30 (8): 1367–1379.e6. April 2020. doi:10.1016/j.cub.2020.01.068. PMID 32243853.
- ↑ "Melanopsin triggers the release of internal calcium stores in response to light". Photochemistry and Photobiology 83 (2): 273–279. March 2007. doi:10.1562/2006-07-11-RA-964. PMID 16961436.
- ↑ "Constitutively active mutants of rhodopsin". Neuron 9 (4): 719–725. October 1992. doi:10.1016/0896-6273(92)90034-b. PMID 1356370.
- ↑ "Synthesis and characterization of a novel retinylamine analog inhibitor of constitutively active rhodopsin mutants found in patients with autosomal dominant retinitis pigmentosa". Proceedings of the National Academy of Sciences of the United States of America 94 (25): 13559–13564. December 1997. doi:10.1073/pnas.94.25.13559. PMID 9391065. Bibcode: 1997PNAS...9413559Y.
- ↑ 43.0 43.1 "Constitutively active rhodopsin and retinal disease". Pharmacology & Therapeutics of Constitutively Active Receptors. Advances in Pharmacology. 70. 2014. pp. 1–36. doi:10.1016/B978-0-12-417197-8.00001-8. ISBN 9780124171978.
- ↑ "A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin". Biophysical Journal 73 (6): 3182–3191. December 1997. doi:10.1016/S0006-3495(97)78344-9. PMID 9414230. Bibcode: 1997BpJ....73.3182M.
- ↑ "Relocating the active-site lysine in rhodopsin and implications for evolution of retinylidene proteins". Proceedings of the National Academy of Sciences of the United States of America 110 (33): 13351–13355. August 2013. doi:10.1073/pnas.1306826110. PMID 23904486. Bibcode: 2013PNAS..11013351D.
- ↑ "Rhodopsin and bacteriorhodopsin: structure-function relationships". FEBS Letters 148 (2): 179–191. November 1982. doi:10.1016/0014-5793(82)80805-3. PMID 6759163.
- ↑ "Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism". Proceedings of the National Academy of Sciences of the United States of America 114 (13): E2608–E2615. March 2017. doi:10.1073/pnas.1617446114. PMID 28289214. Bibcode: 2017PNAS..114E2608G.
- ↑ "Crystallographic analysis of primary visual photochemistry". Angewandte Chemie 45 (26): 4270–4273. June 2006. doi:10.1002/anie.200600595. PMID 16586416.
- ↑ "Quantum mechanical studies on the crystallographic model of bathorhodopsin". Angewandte Chemie 45 (26): 4274–4277. June 2006. doi:10.1002/anie.200600585. PMID 16729349.
- ↑ "The twisted C11=C12 bond of the rhodopsin chromophore--a photochemical hot spot". Journal of the American Chemical Society 129 (35): 10618–10619. September 2007. doi:10.1021/ja071793t. PMID 17691730.
- ↑ "Monomeric G-protein-coupled receptor as a functional unit". Biochemistry 44 (27): 9395–9403. July 2005. doi:10.1021/bi050720o. PMID 15996094.
- ↑ "Rhodopsin Oligomerization and Aggregation". The Journal of Membrane Biology 252 (4–5): 413–423. October 2019. doi:10.1007/s00232-019-00078-1. PMID 31286171.
- ↑ The Nobel Foundation. "The Nobel Prize in Physiology or Medicine 1967". Nobel Media AB 2014. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1967/.
- ↑ "Pre-lumirhodopsin and the bleaching of visual pigments". Nature 197 (Mar 30): 1279–1286. March 1963. doi:10.1038/1971279a0. PMID 14002749. Bibcode: 1963Natur.197.1279Y.
- ↑ "Tautomeric Forms of Metarhodopsin". The Journal of General Physiology 47 (2): 215–240. November 1963. doi:10.1085/jgp.47.2.215. PMID 14080814.
- ↑ "Light-induced protein-protein interactions on the rod photoreceptor disc membrane". Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Biomembranes: A Multi-Volume Treatise. 2. Greenwich, Conn: JAI Press. 1996. pp. 141–198. doi:10.1016/S1874-5342(07)80006-7. ISBN 978-1-55938-659-3.
- ↑ 57.0 57.1 57.2 "Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II". The Journal of Biological Chemistry 278 (5): 3162–3169. January 2003. doi:10.1074/jbc.M209675200. PMID 12427735.
- ↑ "The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation". Journal of Cell Science 115 (Pt 14): 2907–2918. July 2002. doi:10.1242/jcs.115.14.2907. PMID 12082151. https://discovery.ucl.ac.uk/id/eprint/8441/.
- ↑ 59.0 59.1 "Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy". Trends in Molecular Medicine 11 (4): 177–185. April 2005. doi:10.1016/j.molmed.2005.02.007. PMID 15823756.
Further reading
- "On the molecular genetics of retinitis pigmentosa". Science 256 (5058): 804–808. May 1992. doi:10.1126/science.1589761. PMID 1589761. Bibcode: 1992Sci...256..804H.
- "Involvement of cGMP and calcium in the photoresponse in vertebrate photoreceptor cells". The Journal of the Florida Medical Association 82 (7): 485–488. July 1995. PMID 7673885.
- "Rhodopsin mutations in autosomal dominant retinitis pigmentosa". Human Mutation 2 (4): 249–255. 1993. doi:10.1002/humu.1380020403. PMID 8401533.
- "The eye photoreceptor protein rhodopsin. Structural implications for retinal disease". FEBS Letters 528 (1–3): 17–22. September 2002. doi:10.1016/S0014-5793(02)03241-6. PMID 12297272.
- "A completed screen for mutations of the rhodopsin gene in a panel of patients with autosomal dominant retinitis pigmentosa". Human Molecular Genetics 1 (1): 41–45. April 1992. doi:10.1093/hmg/1.1.41. PMID 1301135.
- "Autosomal dominant retinitis pigmentosa: a novel mutation in the rhodopsin gene in the original 3q linked family". Human Molecular Genetics 1 (9): 769–771. December 1992. doi:10.1093/hmg/1.9.769. PMID 1302614.
- "Constitutively active mutants of rhodopsin". Neuron 9 (4): 719–725. October 1992. doi:10.1016/0896-6273(92)90034-B. PMID 1356370.
- "Point mutations of rhodopsin gene found in Japanese families with autosomal dominant retinitis pigmentosa (ADRP)". The Japanese Journal of Human Genetics 37 (2): 125–132. June 1992. doi:10.1007/BF01899733. PMID 1391967.
- "Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa". Neuron 9 (5): 815–830. November 1992. doi:10.1016/0896-6273(92)90236-7. PMID 1418997.
- "A six-generation family with autosomal dominant retinitis pigmentosa and a rhodopsin gene mutation (arginine-135-leucine)". Ophthalmic Paediatrics and Genetics 13 (3): 145–153. September 1992. doi:10.3109/13816819209046483. PMID 1484692.
- "Recombination between rhodopsin and locus D3S47 (C17) in rhodopsin retinitis pigmentosa families". American Journal of Human Genetics 50 (3): 590–597. March 1992. PMID 1539595.
- "Ocular findings associated with a rhodopsin gene codon 106 mutation. Glycine-to-arginine change in autosomal dominant retinitis pigmentosa". Archives of Ophthalmology 110 (5): 646–653. May 1992. doi:10.1001/archopht.1992.01080170068026. PMID 1580841.
- "Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site". Genomics 11 (1): 199–205. September 1991. doi:10.1016/0888-7543(91)90119-Y. PMID 1765377.
- "Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa". Proceedings of the National Academy of Sciences of the United States of America 88 (20): 9370–9374. October 1991. doi:10.1073/pnas.88.20.9370. PMID 1833777. Bibcode: 1991PNAS...88.9370D.
- "Pro-347-Arg mutation of the rhodopsin gene in autosomal dominant retinitis pigmentosa". Genomics 11 (2): 468–470. October 1991. doi:10.1016/0888-7543(91)90159-C. PMID 1840561.
- "Rhodopsin mutations in autosomal dominant retinitis pigmentosa". Proceedings of the National Academy of Sciences of the United States of America 88 (15): 6481–6485. August 1991. doi:10.1073/pnas.88.15.6481. PMID 1862076. Bibcode: 1991PNAS...88.6481S.
- "Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations". American Journal of Ophthalmology 112 (3): 256–271. September 1991. doi:10.1016/s0002-9394(14)76726-1. PMID 1882937.
- "Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis". American Journal of Human Genetics 49 (4): 699–706. October 1991. PMID 1897520.
External links
- Rhodopsin at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Webvision Home Page: The organization of the retina and visual system". University of Utah. 2010-03-01. http://webvision.med.utah.edu/.
- The Rhodopsin Protein
- Photoisomerization of rhodopsin, animation.
- Rhodopsin and the eye, summary with pictures.
 |