Chemistry:Folpet
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-[(Tricloromethyl)sulfanyl]-1H-isoindole-1,3(2H)-dione | |
| Other names
N-(Trichloromethylthio)phthalimide, Orthophaltan, Phaltan, Faltan, Folpan, Faltex, Folpex
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 2588 |
| |
| |
| Properties | |
| C9H4Cl3NO2S | |
| Molar mass | 296.55 g·mol−1 |
| Appearance | white solid |
| Density | 1.72 g/cm3 |
| Melting point | 177 °C (351 °F; 450 K) (decomp.) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H317, H319, H332, H351, H400 | |
| P201, P202, P261, P264, P271, P272, P273, P280, P281, P302+352, P304+312, P304+340, P305+351+338, P308+313, P312, P321, P333+313, P337+313, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
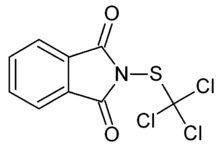
Folpet is the tradename for the organic compound with the formula C6H4(CO)2NSCCl3. It is a fungicide derived from phthalimide (C6H4(CO)2N-) and trichloromethylsulfenyl chloride. The compound is white although commercial samples can appear brownish. It is structurally related to Captan, which is also a trichloromethylsulfenyl-containing fungicide.[1]
Resistance
(As of December 2019) folpet resistance is still unheard of due to its multiple effects.[2] However, in 2001 some degree of cross-resistance was reported in iprodione-resistant South Africa n Botrytis cinerea on grape.[3][4]
References
- ↑ Franz Müller; Peter Ackermann; Paul Margot (2012). "Fungicides, Agricultural, 2. Individual Fungicides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o12_o06. ISBN 978-3-527-30673-2.
- ↑ "Folpet: the essential multi-site fungicide". 2019-12-11. http://www.adama.com/uk/en/news-and-media/adama-news/folpet-the-essential-multi-site-fungicide.
- ↑ Fourie, P.H.; Holz, G. (2001). "Incomplete Cross-Resistance to Folpet and Iprodione in Botrytis cinerea from Grapevine in South Africa". South African Journal of Enology & Viticulture (Stellenbosch University) 22 (1): 3-7. doi:10.21548/22-1-2158. ISSN 2224-7904.
- ↑ Bosch, Frank van den; Oliver, Richard; Berg, Femke van den; Paveley, Neil (2014-08-04). "Governing Principles Can Guide Fungicide-Resistance Management Tactics". Annual Review of Phytopathology (Annual Reviews) 52 (1): 175–195. doi:10.1146/annurev-phyto-102313-050158. ISSN 0066-4286. PMID 24848413.
 |

