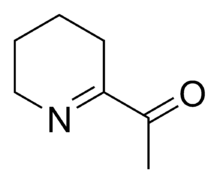
Chemistry:6-Acetyl-2,3,4,5-tetrahydropyridine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(3,4,5,6-Tetrahydropyridin-2-yl)ethan-1-one | |
| Other names
1-(3,4,5,6-Tetrahydropyridin-2-yl)ethanone
2-Acetyl-3,4,5,6-tetrahydropyridine | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 1446593, 1446593 | |
| ChEBI |
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H11NO | |
| Molar mass | 125.171 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
6-Acetyl-2,3,4,5-tetrahydropyridine is an aroma compound and flavor that gives baked goods such as white bread, popcorn, and tortillas their typical smell, together with its structural homolog 2-acetyl-1-pyrroline.
6-Acetyl-2,3,4,5-tetrahydropyridine and 2-acetyl-1-pyrroline are usually formed by Maillard reactions during heating of food. Both compounds have odor thresholds below 0.06 ng/L.[1] [2]
Structure and properties
6-Acetyl-2,3,4,5-tetrahydropyridine is a substituted tetrahydropyridine and a cyclic imine as well as a ketone. The compound exists in a chemical equilibrium with its tautomer 6-acetyl-1,2,3,4-tetrahydropyridine that differs only by the position of the double bond in the tetrahydropyridine ring:
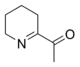
|
[math]\displaystyle{ \rightleftharpoons }[/math] | 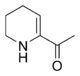
|
| 6-Acetyl-2,3,4,5-tetrahydropyridine | (1 : 2) | 6-Acetyl-1,2,3,4-tetrahydropyridine |
References
- ↑ T. J. Harrison, G. R. Dake (2005). "An expeditious, high-yielding construction of the food aroma compounds 6-acetyl-1,2,3,4-tetrahydropyridine and 2-acetyl-1-pyrroline". J. Org. Chem. 70 (26): 10872–10874. doi:10.1021/jo051940a. PMID 16356012.
- ↑ De Kimpe, Norbert; Stevens, Christian (1993). "A convenient synthesis of 6-acetyl-1,2,3,4-tetrahydropyridine, the principle bread flavor component". Journal of Organic Chemistry 58 (10): 2904–2906. doi:10.1021/jo00062a042.
 |

