Chemistry:Transition metal sulfoxide complex
A transition metal sulfoxide complex is a coordination complex containing one or more sulfoxide ligands. The inventory is large.[2][3]
Scope of sulfoxide ligands
The most common sulfoxide ligand is dimethyl sulfoxide (dmso). Many sulfoxides are known because an enormous range of organic substituents are possible. When the two substituents differ, the ligand is chiral. Chiral sulfoxides are configurationally stable. One example is methyl phenyl sulfoxide.
Structures
 |
|
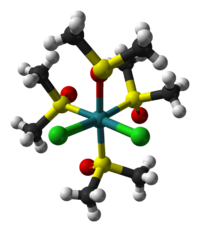
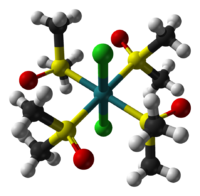
| cis-dichlorotetrakis(dimethylsulfoxide)ruthenium(II) | trans-dichlorotetrakis(dimethylsulfoxide)ruthenium(II) |
Sulfoxides can bind to metals by the oxygen atom or by sulfur. This dichotomy is called linkage isomerism. O-bonded sulfoxide ligands are far more common, especially for 1st row metals. S-bonded sulfoxides are only found for soft metal centers, such as Ru(II). Complexes with both O- and S-bonded sulfoxide ligands are known.[4] In some cases, sulfoxides are bridging ligands, with S bonded to one metal and O bonded to the other.
Synthesis and reactions
Being a polar solvent with a high dielectric constants, dmso dissolves many metal salts to give the corresponding complexes. Other ligand-solvent combinations include acetonitrile and water, which respectively form metal-acetonitrile complexes and metal aquo complexes. Treatment of thioether complexes with peroxide reagents gives sulfoxide complex. In rare cases, sulfoxide complexes are prepared by S-alkylation of sulfenito complexes.[5]
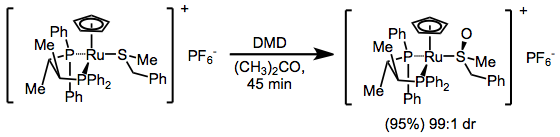
Metal thioether complexes are susceptible to sulfoxidation with dimethyldioxirane.[6]
Reactions
Being weakly basic, sulfoxide ligands are generally labile, i.e. they are rapidly displaced by other more basic ligands.
O-bonded sulfoxide ligands are susceptible to oxidation at sulfur. In this way, the weakly bonded ligand is converted into a leaving group, such as dimethylsulfone. Since dmso is susceptible to deprotonation by strong base, cationic dmso complexes might be expected to undergo H-D exchange under basic conditions. Such behavior is not observed even for the trication [Co(NH
3)
5(dmso)]3+.[8]
Several metal sulfoxide complexes have been investigated as catalysts.[9] The molybdoenzyme DMSO reductase catalyzes the reduction of dmso to dimethyl sulfide.
Examples
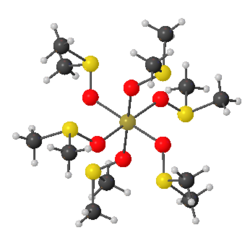
Several homoleptic octahedral complexes of sulfoxides have been characterized by X-ray crystallography. These include the [M(dmso)
6]2+ complexes for M = Cr(III), Mn(II), Fe(II), Fe(III), Co(II), Co(III), Ni(II), Cu(II), Zn(II), Cd(II), and Hg(II). All such derivatives feature O-bonded sulfoxides. The tricationic complex in [Rh(dmso)
6](O
3SCF
3)
3 features one S-bonded and five O-bonded sulfoxide ligands.[10] The complex [Cu(Ph
2SO)
4]2+ is square planar, in contrast to the derivative with dmso ligands. The square planar d8 complex [Rh(dmso)
4]+
features a pairs of S- and O-bonded sulfoxide ligands.[11]
References
- ↑ Lundberg, Daniel; Ullström, Ann-Sofi; d'Angelo, Paola; Persson, Ingmar (2007). "A Structural Study of the Hydrated and the Dimethylsulfoxide, N,N′-Dimethylpropyleneurea, and N,N-Dimethylthioformamide Solvated Iron(II) and Iron(III) Ions in Solution and Solid State". Inorganica Chimica Acta 360 (6): 1809–1818. doi:10.1016/j.ica.2006.09.014.
- ↑ Calligaris, Mario (2004). "Structure and Bonding in Metal Sulfoxide Complexes: An Update". Coordination Chemistry Reviews 248 (3–4): 351–375. doi:10.1016/j.ccr.2004.02.005.
- ↑ Calligaris, M. (1996). "Structure and Bonding in Metal Sulfoxide Complexes". Coordination Chemistry Reviews 153: 83–154. doi:10.1016/0010-8545(95)01193-5.
- ↑ Alessio, Enzo (2004). "Synthesis and Reactivity of Ru-, Os-, Rh-, and Ir-Halide−Sulfoxide Complexes". Chemical Reviews 104 (9): 4203–4242. doi:10.1021/cr0307291. PMID 15352790.
- ↑ Gainsford, Graeme J.; Jackson, William Gregory; Sargeson, Alan M. (1982). "Synthesis of a chiral O-bound sulfoxide from an S-bound sulfenate ion". Journal of the American Chemical Society 104: 137–141. doi:10.1021/ja00365a026.
- ↑ Schenk, W. A.; Steinmetz, B.; Hagel, M.; Adam, W.; Saha-Möller, C. R. Z. Naturforsch. 1997, 1359.
- ↑ Schenk, Wolfdieter A.; Steinmetz, Bernhard; Hagel, Michael; Adam, Waldemar; Saha-Möller, Chantu R. (1997). "Oxyfunctionalization of Allyl Thioether Ruthenium Complexes with Dimethyldioxirane". Zeitschrift für Naturforschung B 52 (11): 1359–1371. doi:10.1515/znb-1997-1114.
- ↑ Dixon, Nicholas E.; Jackson, W. Gregory; Marty, Werner; Sargeson, Alan M. (1982). "Base Hydrolysis of Pentaamminecobalt(II) Complexes of Urea, Dimethyl Sulfoxide and Trimethyl Phosphate". Inorganic Chemistry 21 (2): 688–697. doi:10.1021/ic00132a044.
- ↑ Sipos, Gellért; Drinkel, Emma E.; Dorta, Reto (2015). "The Emergence of Sulfoxides as Efficient Ligands in Transition Metal Catalysis". Chemical Society Reviews 44 (11): 3834–3860. doi:10.1039/C4CS00524D. PMID 25954773. https://api.research-repository.uwa.edu.au/ws/files/9653801/Sulfoxide_review_CSR_2015_revised.pdf.
- ↑ Abbasi, Alireza; Skripkin, Mikhail Yu.; Eriksson, Lars; Torapava, Natallia (2011). "Ambidentate Coordination of Dimethyl Sulfoxide in Rhodium(III) Complexes". Dalton Trans 40 (5): 1111–1118. doi:10.1039/c0dt01026j. PMID 21165499.
- ↑ Dorta, Reto; Rozenberg, Haim; Shimon, Linda J. W.; Milstein, David (2003). "Dimethylsulfoxide as a Ligand for RhI and IrI Complexes—Isolation, Structure, and Reactivity Towards X-H Bonds (X = H, OH, OCH3)". Chemistry - A European Journal 9 (21): 5237–5249. doi:10.1002/chem.200305144. PMID 14613132.
 |