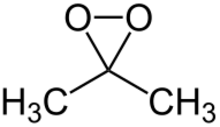
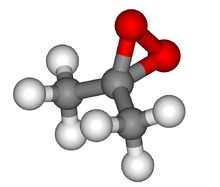
Chemistry:Dimethyldioxirane
| |
| |
 | |
| Names | |
|---|---|
| IUPAC name
3,3-Dimethyldioxirane
| |
| Other names
DMDO
Monoperoxyacetone, Murray's reagent | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyldioxirane (DMDO) is the organic compound with the formula (CH
3)
2CO
2.[1][2] It is the dioxirane derived from acetone and can be considered as a monomer of acetone peroxide. It is a powerful selective oxidizing agent that finds some use in organic synthesis. It is known only in the form of a dilute solution, usually in acetone, and hence the properties of the pure material are largely unknown.[3]
Synthesis
DMDO is not commercially available because of its instability. DMDO can be prepared as dilute solutions (~0.1 M) by treatment of acetone with potassium peroxymonosulfate KHSO5, usually in the form of Oxone (2KHSO5·KHSO4·K2SO4).[4]
The preparation of DMDO is rather inefficient (typical yields < 3%) and typically only yields a relatively dilute solution in acetone (only up to approximately 0.1 M). This is tolerable as preparation uses inexpensive substances: acetone, sodium bicarbonate, and potassium peroxymonosulfate (commercially known as "oxone"). The solution can be stored at low temperatures and its concentration may be assayed immediately prior to its use.
The more active compound methyl(trifluoromethyl)dioxirane (H3C)(F3C)CO2 can be similarly prepared from methyl trifluoromethyl ketone.
Stability
Cold solutions (−10 to −20 °C) are stable for days. Decomposition is accelerated by light and heavy metals.[3]
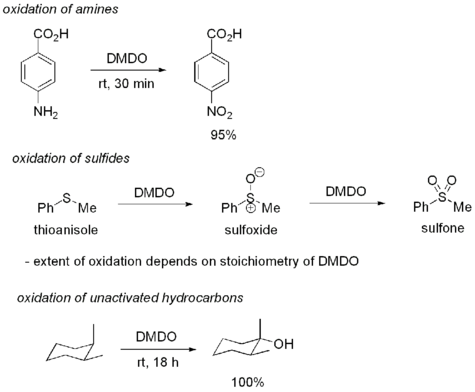
Uses
DMDO is most commonly used for the oxidation of alkenes to epoxides. One advantage of using DMDO is that the only byproduct of oxidation is acetone, a fairly innocuous and volatile compound. DMDO oxidations are particularly mild, sometimes allowing oxidations which might not otherwise be possible.
Despite its high reactivity, DMDO displays good selectivity for electron-rich olefins. DMDO will also oxidize several other functional groups. For example, DMDO will oxidize primary amines to nitro compounds and sulfides to sulfoxides. In some cases, DMDO will even oxidize unactivated C-H bonds:
DMDO can also be used to convert nitro compounds to carbonyl compounds (Nef reaction).[5]
See also
References
- ↑ "Robert W. Murray Biography". University of Missouri–St. Louis. http://www.umsl.edu/chemistry/Seminar%20Programs/murraybio.html. Retrieved 14 October 2015.
- ↑ Murray, Robert W. (July 1989). "Chemistry of dioxiranes. 12. Dioxiranes". Chemical Reviews 89 (5): 1187–1201. doi:10.1021/cr00095a013.
- ↑ 3.0 3.1 Crandall, J. K.; Curc, R; D'Accolti, L; Fusco, C (15 Oct 2005). "Dimethyldioxirane". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd329.pub2. ISBN 0471936235.
- ↑ Robert W. Murray and Megh Singh (1988). "Synthesis of epoxides using dimethyldioxirane: trans-stilbene oxide"]. Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv9p0288.; Collective Volume, 9, pp. 288
- ↑ Adam, Waldemar; Makosza, Mieczyslaw; Saha-Möller, Chantu R.; Zhao, Cong-Gui (1998). "A Mild and Efficient Nef Reaction for the Conversion of Nitro to Carbonyl Group by Dimethyldioxirane (DMD) Oxidation of Nitronate Anions". Synlett 1998 (12): 1335–1336. doi:10.1055/s-1998-1947.
 |