Chemistry:Kojibiose
From HandWiki
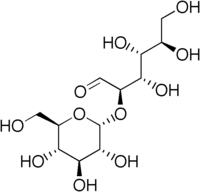
| |
| Names | |
|---|---|
| IUPAC name
2-O-α-D-Glucopyranosyl-D-glucose
| |
| Systematic IUPAC name
(2R,3S,4R,5R)-3,4,5,6-tetrahydroxy-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexanal | |
| Other names
2-alpha-D-glucosyl-D-glucose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Kojibiose |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
| Density | 1.688 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Kojibiose is a disaccharide. It is a product of the caramelization of glucose.[1] It is also present in honey (approx. 3%).[2]
Kojibiose has a mild sweet taste, but low calorie count. In combination with its prebiotic properties, kojibiose could function as a sugar substitute. However, kojibiose is hard to synthesize on an industrial scale. Recently, two enzyme approaches transforming sucrose and lactose or sucrose and glucose into kojibiose have been developed, potentially solving the synthetization problem. [3] [4]
References
- ↑ Sugisawa, Hirqshi; Edo, Hiroshi (1966). "The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose". Journal of Food Science 31 (4): 561. doi:10.1111/j.1365-2621.1966.tb01905.x.
- ↑ Siddiqua, I.R; Furgala, B (1967). "Isolation and characterization of oligosaccharides from honey". Journal of Apicultural Research 6 (3): 139–145. doi:10.1080/00218839.1967.11100174.
- ↑ Diez-Municio, Marina; Montilla, Antonia; Moreno, F. Javier; Herrero, Miguel (2014). "A sustainable biotechnological process for the efficient synthesis of kojibiose". Green Chemistry 16 (4): 2219–2226. doi:10.1039/C3GC42246A.
- ↑ Verhaeghe, Tom; De Winter, Karel (2016). "Converting bulk sugars into prebiotics: semi-rational design of a transglucosylase with controlled selectivity". Chemical Communications 52 (18): 2687–3689. doi:10.1039/C5CC09940D. PMID 26858011.
 |

