Chemistry:Helenin
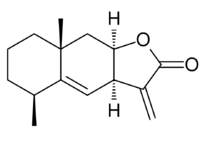 Alantolactone
| |
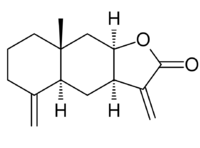 Isoalantolactone
| |
| Names | |
|---|---|
| IUPAC names
Alantolactone: (3aR,5S,8aR,9aR)-5,8a-Dimethyl-3-methylene-3a,5,6,7,8,8a,9,9a-octahydronaphtho[2,3-b]furan-2(3H)-one
Isoalantolactone: (3aR,4aS,8aR,9aR)-8a-Methyl-3,5-bis(methylene)decahydronaphtho[2,3-b]furan-2(3H)-one | |
| Other names
Elecampane camphor, Inula camphor, Alant camphor
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C15H20O2 | |
| Molar mass | 232.323 g·mol−1 |
| Appearance | Crystalline powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Helenin is a phytochemical mixture found in many plant species, including the Inula helenium (elecampane) of the family Asteraceae. It is a mixture of two isomeric sesquiterpene lactones, alantolactone and isoalantolactone.
In 1895 the German scientists Julius Bredt and Wilhelm Posh extracted helenin from Inula helenium and determined its physical and chemical properties.[1]
Natural sources
Alantolactone occurs in the roots of Inula helenium and other Inula species.[2]
Properties
Helenin can be extracted from the roots of Inula helenium using alcohol or other non-polar solvents to produce a mixture with a composition of about 40% alantolactone and 60% isoalantolactone.[3]
Biological activity
Alantolactone has a variety of in vitro biochemical properties, including:
- induces apoptosis and cell cycle arrest in lung squamous cancer cells[4]
- suppresses STAT3 activation[5]
- has antiinflammatory effects by inhibiting chemokine production and STAT1 phosphorylation[6]
- anti-fungal[7]
- anti-microbial[8]
Toxicity
Certain individuals have experienced contact dermatitis when exposed to alantolactone.[9]
References
- ↑ Chemical Society (Great Britain) (1895). The collected works of Sir Humphry Davy ...: Discourses delivered before the Royal society. Elements of agricultural chemistry, pt. I. Smith, elder and Company. pp. 555. https://books.google.com/books?id=ksdLAAAAYAAJ&pg=PA555. Retrieved 31 July 2015.
- ↑ Hoffmann, David (2003). Medical Herbalism:The Science and Practice of Herbal Medicine. Health & Fitness. ISBN 978-1594778902. https://books.google.com/books?isbn=1594778906.
- ↑ Xu, Renjie (2014). "Pharmacokinetic Comparison of Isoalantolactone and Alantolactone in Rats after Administration Separately by Optimization of an UPLC-MS2 Method". Journal of Chemistry 2014: 1–8. doi:10.1155/2014/354618.
- ↑ Zhao, Peng (19 Jan 2015). "Alantolactone Induces Apoptosis and Cell Cycle Arrest on Lung Squamous Cancer SK-MES-1 Cells". Journal of Biochemical and Molecular Toxicology 29 (5): 199–226. doi:10.1002/jbt.21685. PMID 25597476.
- ↑ Chun, J (1 Feb 2015). "Alantolactone selectively suppresses STAT3 activation and exhibits potent anticancer activity in MDA-MB-231 cells.". Cancer Letters 357 (1): 393–403. doi:10.1016/j.canlet.2014.11.049. PMID 25434800. https://zenodo.org/record/851358.
- ↑ Hye, Sun Lim (17 Apr 2015). "Alantolactone from Saussurea lappa Exerts Antiinflammatory Effects by Inhibiting Chemokine Production and STAT1 Phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells". Phytotherapy Research 29 (7): 1088–1096. doi:10.1002/ptr.5354. PMID 25881570.
- ↑ Alejandro, Barreroa (2000). "New sources and antifungal activity of sesquiterpene lactones". Fitoterapia 66 (71): 60–64. doi:10.1016/s0367-326x(99)00122-7. PMID 20095126.
- ↑ O'Shea, S (2009). "In vitro activity of Inula helenium against clinical Staphylococcus aureus strains including MRSA". British Journal of Medical Science 66 (4): 186–9. doi:10.1080/09674845.2009.11730271. PMID 20095126.
- ↑ Stampf, J (August 1978). "Allergic contact dermatitis due to sesquiterpene lactones. A comparative study of human and animal sensitivity to alpha-methylene-gamma-butyrolactone and derivatives". The British Journal of Dermatology 99 (2): 163–9. doi:10.1111/j.1365-2133.1978.tb01977.x. PMID 698105.
 |

