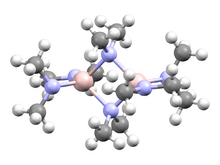
Chemistry:Tris(dimethylamino)aluminium dimer
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
N-[bis(dimethylamino)alumanyl]-N-methylmethanamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H36Al2N6 | |
| Molar mass | 318.425 g·mol−1 |
| Appearance | Colorless solid |
| Density | 0.865 g/cm3 |
| Melting point | 82-84 °C |
| Boiling point | 90 °C |
| Hazards[1] | |
| Main hazards | Flammable, Corrosive |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H260, H314 | |
| P223, P231+232, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P335+334, P363, P370+378, P402+404, P405, P501 | |
| Flash point | 21.1 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tris(dimethylamino)aluminium dimer, formally bis(μ-dimethylamino)tetrakis(dimethylamino)dialuminium, is an amide complex of aluminium. This compound may be used as a precursor to other aluminium complexes.
Commercially available, this compound may be prepared from lithium dimethylamide and aluminium trichloride.[2]
References
- ↑ "Tris(dimethylamido)aluminum(III)". American Elements. https://www.americanelements.com/tris-dimethylamido-aluminum-iii-32093-39-3. Retrieved August 21, 2019.
- ↑ Waggoner, K.M.; Olmstead, M.M.; Power, P.P. (1990). "Structural and spectroscopic characterization of the compounds [Al(NMe2)3]2, [Ga(NMe2)3]2, [(Me2N)2Al{μ-N(H)1-Ad}]2 (1-Ad = 1-adamantanyl) and [{Me(μ-NPh2)Al}2NPh(μ-C6H4)]". Polyhedron 9 (2–3): 257–263. doi:10.1016/S0277-5387(00)80578-1.
 |

