Biology:Guanylin
| guanylate cyclase activator 2A (guanylin) | |
|---|---|
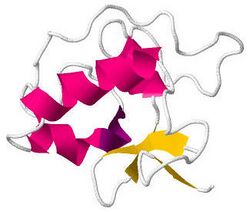 Solution structure of human proguanylin.[1] | |
| Identifiers | |
| Symbol | GUCA2A |
| Alt. symbols | GUCA2 |
| NCBI gene | 2980 |
| HGNC | 4682 |
| OMIM | 139392 |
| PDB | 1O8R |
| RefSeq | NM_033553 |
| UniProt | Q02747 |
| Other data | |
| Locus | Chr. 1 p35-p34 |
| Guanylin precursor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbol | Guanylin | ||||||||||
| Pfam | PF02058 | ||||||||||
| InterPro | IPR000879 | ||||||||||
| SCOP2 | 1uyb / SCOPe / SUPFAM | ||||||||||
| |||||||||||
Guanylin is a 15 amino acid peptide that is secreted by goblet cells in the colon. Guanylin acts as an agonist of the guanylyl cyclase receptor GC-C and regulates electrolyte and water transport in intestinal and renal epithelia.[2][3] Upon receptor binding, guanylin increases the intracellular concentration of cGMP, induces chloride secretion and decreases intestinal fluid absorption, ultimately causing diarrhoea.[4] The peptide stimulates the enzyme through the same receptor binding region as the heat-stable enterotoxins.[3]
Researches have found that a loss in guanylin expression can lead to colorectal cancer due to guanylyl cyclase C's function as an intestinal tumor suppressor.[5] When guanylin expression was measured on over 250 colon cancer patients, more than 85% of patients had a loss of guanylin expression in cancerous tissue samples by 100-1000 times when compared to the same patients's nearby healthy colon tissue.[5] Another study done on genetically engineered mice found that mice on a high calorie diet had reduced guanylin expression in the colon.[6] This loss of expression then resulted in guanylyl cyclase C inhibition and the formation of tumors, therefore linking diet-induced obesity with colorectal cancer. [6]
Human proteins containing this domain
GUCA2A; GUCA2B;
Structure
This peptide has two topogies,[7] both isoforms are shown below:
 Structure of the A-form of human uroguanylin.[7] |
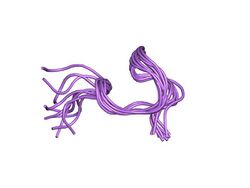 Structure of the B-form of human uroguanylin.[7] |
References
- ↑ PDB: 1O8R; "Solution structure of human proguanylin: the role of a hormone prosequence". The Journal of Biological Chemistry 278 (26): 24118–24. June 2003. doi:10.1074/jbc.M300370200. PMID 12707255.
- ↑ "Genomic sequence of the murine guanylin gene". Genomics 24 (3): 583–7. December 1994. doi:10.1006/geno.1994.1670. PMID 7713512.
- ↑ 3.0 3.1 "Precursor structure, expression, and tissue distribution of human guanylin". Proceedings of the National Academy of Sciences of the United States of America 89 (19): 9089–93. October 1992. doi:10.1073/pnas.89.19.9089. PMID 1409606. Bibcode: 1992PNAS...89.9089D.
- ↑ "Guanylin: an endogenous activator of intestinal guanylate cyclase". Proceedings of the National Academy of Sciences of the United States of America 89 (3): 947–51. February 1992. doi:10.1073/pnas.89.3.947. PMID 1346555. Bibcode: 1992PNAS...89..947C.
- ↑ 5.0 5.1 "Guanylin hormone loss could trigger colon cancer". The Lancet. Oncology 15 (12): e537. November 2014. doi:10.1016/s1470-2045(14)71032-0. PMID 25602115.
- ↑ 6.0 6.1 "Obesity-Induced Colorectal Cancer Is Driven by Caloric Silencing of the Guanylin-GUCY2C Paracrine Signaling Axis". Cancer Research 76 (2): 339–46. January 2016. doi:10.1158/0008-5472.CAN-15-1467-T. PMID 26773096.
- ↑ 7.0 7.1 7.2 "One peptide, two topologies: structure and interconversion dynamics of human uroguanylin isomers". The Journal of Peptide Research 52 (3): 229–40. September 1998. doi:10.1111/j.1399-3011.1998.tb01480.x. PMID 9774236.
External links
- guanylin at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

