Biology:Mucor racemosus
| Mucor racemosus | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Mucoromycota |
| Order: | Mucorales |
| Family: | Mucoraceae |
| Genus: | Mucor |
| Species: | M. racemosus
|
| Binomial name | |
| Mucor racemosus Bull. (1791)
| |
| Synonyms | |
| |
Mucor racemosus is a rapidly growing, weedy mould belonging to the division Mucoromycota.[1] It is one of the earliest fungi to be grown in pure culture and was first isolated in 1886.[citation needed] It has a worldwide distribution and colonizes many habitats such as vegetational products, soil and houses.[2][3] The fungus is mostly known for its ability to exhibit both filamentous and yeast-like morphologies, often referred to as dimorphism.[2] Stark differences are seen in both forms and conditions of the environment heavily affect the phases of the M. racemosus.[2] Like many fungi, it also reproduces both sexually and asexually.[2] The dimorphic capacity of this species has been proposed as an important factor in its pathogenicity and has enhanced the industrial importance. This species is considered an opportunistic pathogen, generally limited to immunocompromised individuals.[4] It also been associated with allergy and inflammations of facial sinuses.[4] Its association with allergy has made it a common fungus used in allergen medical testing.[5][6] Industrial use of the fungus is in the production of enzymes and the manufacture of certain dairy foods.[7][8][9]
Morphology and taxonomy
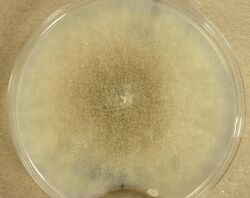
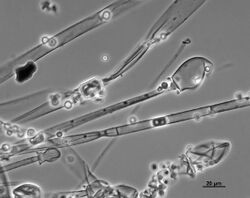
The dimorphic form of the species mainly exists and grows vegetatively as either a filamentous hyphae (mould form) or as spherical yeast (yeast form).[2] However, the organism is best known from the mould form which is characterised by the production of asexual reproductive state consisting of tall (up to 2 cm) needle-like sporangiophores with an apical swelling enclosed by a large sporangium filled with ellipsoidal, single-celled, smooth-walled, unpigmented sporangiospores.[citation needed] In the laboratory, the fungus forms dark grey or light grey colonies on most common laboratory media.[citation needed] If subjected to anaerobic conditions, the fungus may convert to the yeast-like form.[2] Anaerobic conditions and 30% carbon dioxide presence stimulate conversion to yeast form. Likewise, cultures supplemented with Tween 80, ergosterol and supplied with 100% nitrogen also converted to yeast.[10] Conversely, increasing oxygen concentration will cause conversion of the yeast form to the mould form.[2] Like many zygomycetes, M. racemosus reproduces both sexually and asexually depending on environmental conditions. During sexual reproduction, hyphae of compatible mating types touch and fuse, ultimately giving rise to a thick-walled zygosporangium containing a single zygospore. Germination from the zygospore leads to growth of new hyphae that give rise to asexual spores of both + and - mating type.[2] Germination of these spores produces new haploid hyphae of the same mating type.[2]
Physiology and ecology
M. racemosus possesses the ability to exhibit multiple morphology (mainly, filamentous and spherical shape) to withstand various environmental stress.[11] This has given it ability to survive many conditions and it has a worldwide distribution, reported most frequently in Europe as well as Americas.[citation needed] In the tropics, it has been seen at higher altitudes.[citation needed] While the species is primarily soil-based, it has been shown to exist elsewhere such as in horse manure, plant remains, grains, vegetables and nuts.[2] Typically, it is often seen on plant-based materials such as soft fruit, fruit juice and marmalade[citation needed] but it has also been isolated from non-plant sources like soft camembert cheese.[citation needed] M. racemosus has also been isolated from the human gut microbiome of non-obese individuals.[12] It is the most common mould found in the floor dust in houses and is largely considered as an indoor mould.[3]M. racemosus is uniquely known for its ability to display multiple morphologies but most of the time, studies are made based on the dimorphic form of the species.[11] It is a facultative anaerobic zygomycote and fast-growing, conferring it ability to survive in multiple conditions/locations all over the world.[2][11] M. racemosus possesses the ability to biosynthesize chitin and chitosan, which has been proposed as a mechanism supporting the ability of the fungus to switch between the yeast and the mould phases.[13] Genomic analysis of M. racemosus has revealed genes similar to human RAS genes, and it is proposed that these genes help with germination and dimorphism.[14][15] Protein kinase A (PKA) genes such as pkaR are highly also expressed during dimorphic shift.[16]
Human disease
M. racemosus is a rare agent of human disease, typically only associated with opportunistic infection of immunocompromised individuals such as children, elderly and diseased patients (HIV, Ebola etc.).[11] It is an agent of Mucormycosis, a potentially life-threatening infection often involving the head airways.[4] Pulmonary, cutaneous, and gastrointestinal (GI) infections have also been observed leading to an array of clinical presentations in infected individuals. Risk factors such as diabetic ketoacidosis and neutropenia are present in most cases.[4] Treatment of M. racemosus can be difficult due to histopathologic differentiation of the fungus.[1] In addition to commonly used antifungal agents, biological compounds like Lovastatin, Aleuria aurantia lectin (AAL) and antimicrobial peptides (AMPs LR14) have been isolated and showed antimicrobial effects towards M. racemosus.[17][18][19] Allergies to M. racemosus have been reported to affect immunologically normal individuals from in a range of places (Netherlands, Turkey and Brazil).[20][21][22] Allergy to M. racemosus has been also associated with fungal rhinosinusitis,[23] rhinitis and extrinsic allergic alveolitis.[24][25] Asthmatic patients have also shown elevated sensitization to M. racemosus.[26] Mucor racemosus-specific IgE antibody is commonly used and available for medical as well as laboratory use in allergen assay (ImmunoCAP).[5][6]
Commercial and biotechnological use
The capacity of M. racemosus to grow as a yeast and its various abilities to manufacture biochemicals have led to its use in industry. For example, it can produce a high yield of phytase, an important industrial enzyme.[7][8] It also has an increased extracellular protease activity, suggesting its biotechnological suitability for the production of other industrial enzymes.[7][8] In the manufacture of sufu (fermented cheese-like soybean product common in China and Vietnam), the fungal fermentation of soybean curd (tofu) results in moulded tofu, pehtze. The final product (sufu) is obtained by maturing pehtze in a brine containing alcohol and salt for several months.[9]
It possesses the ability to adapt phenotypically to several different antibiotics after exposure to a single drug, which makes it a good model for phenotypic multidrug resistance in lower eukaryotes. It has been shown to adapt to famous antibiotics like cycloheximide, trichodermin and amphotericin B.[2][27] Cells adapted to cycloheximide particularly have been observed to be 40-times more resistant than non-adapted cells to the drug. These adapted cells have been studied to better understand their greater efficiency of membrane transport (efflux of drugs).[28]
Mucor racemosus can biotransform lipids like 4-ene-3-one steroids and 20(S)-Protopanaxatriol into several different products, some of which have anticancer properties (as the metabolites resulted in increased intracellular calcium ion content, leading to cell cycle arrest and apoptosis).[29][30] Two of the products formed from this biotransformation are two novel hydroperoxylated metabolites that have been shown to be effective against prostate cancer cells.[31] Secondary metabolites of M. racemosus do not exhibit genotoxic activity, and the species is not known to be a producer of mycotoxins. However, some secondary metabolites of the fungus have been found to have anti-inflammatory activity similar to the drug dexamethasone .[32]
References
- ↑ 1.0 1.1 Hata, DJ; Buckwalter, SP; Pritt, BS; Roberts, GD; Wengenack, NL (July 2008). "Real-time PCR method for detection of zygomycetes.". Journal of Clinical Microbiology 46 (7): 2353–8. doi:10.1128/jcm.02331-07. PMID 18480229.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Current research, technology and education topics in applied microbiology and microbial biotechnology. Badajoz, Spain: Formatex Research Center. 2010. pp. 201–212. ISBN 978-84-614-6194-3.
- ↑ 3.0 3.1 Abdel-Hafez, SI; Shoreit, AA (November 1985). "Mycotoxins producing fungi and mycoflora of air-dust from Taif, Saudi Arabia.". Mycopathologia 92 (2): 65–71. doi:10.1007/bf00444085. PMID 3935928.
- ↑ 4.0 4.1 4.2 4.3 Nancy, Crum-Cianflone (2018-10-04). Mucormycosis. WebMD. http://emedicine.medscape.com/article/222551-overview.
- ↑ 5.0 5.1 "Mucor Racemosus IgE". http://www.viracoribt.com/Test-Catalog/Detail/Mucor-racemosus-IgE--75610S.
- ↑ 6.0 6.1 "Allergen, Fungi and Molds, Mucor racemosus". http://ltd.aruplab.com/Tests/Pub/0055442.
- ↑ 7.0 7.1 7.2 Bogar, B.; Szakacs, G.; Pandey, A.; Abdulhameed, S.; Linden, J.C.; Tengerdy, R.P. (4 April 2003). "Production of Phytase by Mucor racemosus in Solid-State Fermentation". Biotechnology Progress 19 (2): 312–319. doi:10.1021/bp020126v. PMID 12675565.
- ↑ 8.0 8.1 8.2 Alves, MH; de Campos-Takaki, GM; Okada, K; Ferreira-Pessoa, IH; Milanez, AI (June 2005). "[Detection of extracellular protease in Mucor species].". Revista Iberoamericana de Micologia 22 (2): 114–7. doi:10.1016/s1130-1406(05)70020-6. PMID 16107171.
- ↑ 9.0 9.1 Han, BZ; Kuijpers, AF; Thanh, NV; Nout, MJ (April 2004). "Mucoraceous moulds involved in the commercial fermentation of Sufu Pehtze.". Antonie van Leeuwenhoek 85 (3): 253–7. doi:10.1023/b:anto.0000020157.72415.b9. PMID 15028872. https://library.wur.nl/WebQuery/wurpubs/337359.
- ↑ Lübbehüsen, TL; Nielsen, J; McIntyre, M (February 2003). "Morphology and physiology of the dimorphic fungus Mucor circinelloides (syn. M. racemosus) during anaerobic growth.". Mycological Research 107 (Pt 2): 223–30. doi:10.1017/s0953756203007299. PMID 12747334. https://pdfs.semanticscholar.org/1a22/36340a20145840907713b625c1d9561ec003.pdf.
- ↑ 11.0 11.1 11.2 11.3 Inderlied, Clark; Peters, Julius; Cihlar, Ronald (1985). Fungal Dimorphism With Emphasis on Fungi Pathogenic for Humans. Boston, MA: Springer US. pp. 337–359. ISBN 978-1-4684-4982-2.
- ↑ Mar Rodríguez, M; Pérez, D; Javier Chaves, F; Esteve, E; Marin-Garcia, P; Xifra, G; Vendrell, J; Jové, M et al. (12 October 2015). "Obesity changes the human gut mycobiome.". Scientific Reports 5: 14600. doi:10.1038/srep14600. PMID 26455903.
- ↑ Domek, DB; Borgia, PT (June 1981). "Changes in the rate of chitin-plus-chitosan synthesis accompany morphogenesis of Mucor racemosus.". Journal of Bacteriology 146 (3): 945–51. doi:10.1128/jb.146.3.945-951.1981. PMID 7240089.
- ↑ Casale, WL; Mcconnell, DG; Wang, SY; Lee, YJ; Linz, JE (December 1990). "Expression of a gene family in the dimorphic fungus Mucor racemosus which exhibits striking similarity to human ras genes.". Molecular and Cellular Biology 10 (12): 6654–63. doi:10.1128/mcb.10.12.6654. PMID 1701021.
- ↑ Roze, LV; Mahanti, N; Mehigh, R; McConnell, DG; Linz, JE (December 1999). "Evidence that MRas1 and MRas3 proteins are associated with distinct cellular functions during growth and morphogenesis in the fungus Mucor racemosus.". Fungal Genetics and Biology 28 (3): 171–89. doi:10.1006/fgbi.1999.1177. PMID 10669583.
- ↑ Wolff, AM; Appel, KF; Petersen, JB; Poulsen, U; Arnau, J (May 2002). "Identification and analysis of genes involved in the control of dimorphism in Mucor circinelloides (syn. racemosus).". FEMS Yeast Research 2 (2): 203–13. doi:10.1016/s1567-1356(02)00090-9. PMID 12702308.
- ↑ Amano, K; Katayama, H; Saito, A; Ando, A; Nagata, Y (2012). "Aleuria aurantia lectin exhibits antifungal activity against Mucor racemosus.". Bioscience, Biotechnology, and Biochemistry 76 (5): 967–70. doi:10.1271/bbb.110982. PMID 22738968.
- ↑ Gupta, R; Srivastava, S (September 2014). "Antifungal effect of antimicrobial peptides (AMPs LR14) derived from Lactobacillus plantarum strain LR/14 and their applications in prevention of grain spoilage.". Food Microbiology 42: 1–7. doi:10.1016/j.fm.2014.02.005. PMID 24929709.
- ↑ Roze, LV; Linz, JE (November 1998). "Lovastatin triggers an apoptosis-like cell death process in the fungus Mucor racemosus.". Fungal Genetics and Biology 25 (2): 119–33. doi:10.1006/fgbi.1998.1093. PMID 9974223.
- ↑ Beaumont, F; Kauffman, HF; de Monchy, JG; Sluiter, HJ; de Vries, K (April 1985). "Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. II. Comparison of aerobiological data and skin tests with mould extracts in an asthmatic population.". Allergy 40 (3): 181–6. doi:10.1111/j.1398-9995.1985.tb00214.x. PMID 4039540.
- ↑ Güneser, S; Atici, A; Köksal, F; Yaman, A (1994). "Mold allergy in Adana, Turkey.". Allergologia et Immunopathologia 22 (2): 52–4. PMID 8059675.
- ↑ Mohovic, J; Gambale, W; Croce, J (1998). "Cutaneous positivity in patients with respiratory allergies to 42 allergenic extracts of airborne fungi isolated in São Paulo, Brazil.". Allergologia et Immunopathologia 16 (6): 397–402. PMID 3242377.
- ↑ Zhao, Z; Li, L; Wan, Z; Chen, W; Liu, H; Li, R (April 2011). "Simultaneous detection and identification of Aspergillus and mucorales species in tissues collected from patients with fungal rhinosinusitis.". Journal of Clinical Microbiology 49 (4): 1501–7. doi:10.1128/jcm.02262-10. PMID 21325541.
- ↑ Koschel, D; Sennekamp, J; Schurz, C; Müller-Wening, D (September 2004). "[Misting-fountain-alveolitis].". Pneumologie (Stuttgart, Germany) 58 (9): 666–9. doi:10.1055/s-2004-830044. PMID 15343489.
- ↑ Namysłowski, G; Rogala, B; Mrówka-Kata, K; Ponińska-Polańczuk, J (1998). "[The role of imperfect fungi in etiopathogenesis of allergic rhinitis].". Otolaryngologia Polska. The Polish Otolaryngology 52 (3): 277–80. PMID 9760768.
- ↑ Soeria-Atmadja, D; Onell, A; Kober, A; Matsson, P; Gustafsson, MG; Hammerling, U (December 2007). "Multivariate statistical analysis of large-scale IgE antibody measurements reveals allergen extract relationships in sensitized individuals.". The Journal of Allergy and Clinical Immunology 120 (6): 1433–40. doi:10.1016/j.jaci.2007.07.021. PMID 17825892.
- ↑ Leathers, TD; Sypherd, PS (June 1985). "Inducible phenotypic multidrug resistance in the fungus Mucor racemosus.". Antimicrobial Agents and Chemotherapy 27 (6): 892–6. doi:10.1128/aac.27.6.892. PMID 4026262.
- ↑ Shearer G, Jr; Sypherd, PS (March 1988). "Cycloheximide efflux in antibiotic-adapted cells of the fungus Mucor racemosus.". Antimicrobial Agents and Chemotherapy 32 (3): 341–5. doi:10.1128/aac.32.3.341. PMID 3364951.
- ↑ Ge, WZ; Li, N; Shan, LH; Liu, HM (June 2007). "[Microbial transformation of 4-ene-3-one steroids by Mucor racemosus].". Wei Sheng Wu Xue Bao = Acta Microbiologica Sinica 47 (3): 540–3. PMID 17672323.
- ↑ Chen, G; Yang, X; Nong, S; Yang, M; Xu, B; Zhang, W (March 2013). "Two novel hydroperoxylated products of 20(S)-protopanaxadiol produced by Mucor racemosus and their cytotoxic activities against human prostate cancer cells.". Biotechnology Letters 35 (3): 439–43. doi:10.1007/s10529-012-1098-x. PMID 23183919.
- ↑ Chen, G; Ge, H; Song, Y; Li, J; Zhai, X; Wu, J; Ling, X (October 2015). "Biotransformation of 20(S)-protopanaxatriol by Mucor racemosus and the anti-cancer activities of some products.". Biotechnology Letters 37 (10): 2005–9. doi:10.1007/s10529-015-1877-2. PMID 26054722.
- ↑ Meier, SM; Muqaku, B; Ullmann, R; Bileck, A; Kreutz, D; Mader, JC; Knasmüller, S; Gerner, C (2015). "Proteomic and Metabolomic Analyses Reveal Contrasting Anti-Inflammatory Effects of an Extract of Mucor Racemosus Secondary Metabolites Compared to Dexamethasone.". PLOS ONE 10 (10): e0140367. doi:10.1371/journal.pone.0140367. PMID 26496078.
Wikidata ☰ Q2450163 entry
 |