Biology:Lovastatin
 | |
| Clinical data | |
|---|---|
| Trade names | Mevacor, Altocor, others |
| Other names | Monacolin K, Mevinolin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688006 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <5%[1] |
| Protein binding | >98%[1] |
| Metabolism | Liver (CYP3A and CYP2C8 substrate)[1] |
| Elimination half-life | 2–5 hours[1] |
| Excretion | Faeces (83%), urine (10%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H36O5 |
| Molar mass | 404.547 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease.[2] Its use is recommended together with lifestyle changes.[2] It is taken by mouth.[2]
Common side effects include diarrhea, constipation, headache, muscles pains, rash, and trouble sleeping.[2] Serious side effects may include liver problems, muscle breakdown, and kidney failure.[2] Use during pregnancy may harm the baby and use during breastfeeding is not recommended.[3] It works by decreasing the liver's ability to produce cholesterol by blocking the enzyme HMG-CoA reductase.[2]
Lovastatin was patented in 1979 and approved for medical use in 1987.[4] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[2] In 2020, it was the 99th most commonly prescribed medication in the United States, with more than 7 million prescriptions.[6][7]
Medical uses
The primary uses of lovastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.[8] It is recommended to be used only after other measures, such as diet, exercise, and weight reduction, have not improved cholesterol levels.[8]
Side effects
Lovastatin is usually well tolerated, with the most common side effects being, in approximately descending order of frequency: creatine phosphokinase elevation, flatulence, abdominal pain, constipation, diarrhoea, muscle aches or pains, nausea, indigestion, weakness, blurred vision, rash, dizziness and muscle cramps.[9] As with all statin drugs, it can rarely cause myopathy, hepatotoxicity (liver damage), dermatomyositis or rhabdomyolysis.[9] This can be life-threatening if not recognised and treated in time, so any unexplained muscle pain or weakness whilst on lovastatin should be promptly mentioned to the prescribing doctor. Other uncommon side effects that should be promptly mentioned to either the prescribing doctor or an emergency medical service include:[10]
These less serious side effects should still be reported if they persist or increase in severity:[10]
Contraindications
Contraindications, conditions that warrant withholding treatment with lovastatin, include pregnancy, breast feeding, and liver disease. Lovastatin is contraindicated during pregnancy (Pregnancy Category X); it may cause birth defects such as skeletal deformities or learning disabilities. Owing to its potential to disrupt infant lipid metabolism, lovastatin should not be taken while breastfeeding.[11] Patients with liver disease should not take lovastatin.[12]
Interactions
As with atorvastatin, simvastatin, and other statin drugs metabolized via CYP3A4, drinking grapefruit juice during lovastatin therapy may increase the risk of side effects. Components of grapefruit juice, the flavonoid naringin, or the furanocoumarin bergamottin inhibit CYP3A4 in vitro,[13] and may account for the in vivo effect of grapefruit juice concentrate decreasing the metabolic clearance of lovastatin, and increasing its plasma concentrations.[14]
Mechanism of action
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate.[15] Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA, which binds to the HMG-CoA reductase. Lovastatin is a prodrug, an inactive lactone in its native form, the gamma-lactone closed ring form in which it is administered, is hydrolysed in vivo to the β-hydroxy acid open ring form; which is the active form.
Lovastatin and other statins have been studied for their chemopreventive and chemotherapeutic effects. No such effects were seen in the early studies.[16] More recent investigations revealed some chemopreventive and therapeutic effects, for certain types of cancer, especially in combination of statins with other anticancer drugs.[17] It is likely that these effect are mediated by the properties of statins to reduce proteasome activity, leading to an accumulation of cyclin-dependent kinase inhibitors p21 and p27, and to subsequent G1-phase arrest, as seen in cells of different cancer lines.[18][19]
History

Compactin and lovastatin, natural products with a powerful inhibitory effect on HMG-CoA reductase, were discovered in the 1970s, and taken into clinical development as potential drugs for lowering LDL cholesterol.[21][22]
In 1982, some small-scale clinical investigations of lovastatin, a polyketide-derived natural product isolated from Aspergillus terreus, in very high-risk patients were undertaken, in which dramatic reductions in LDL cholesterol were observed, with very few adverse effects. After the additional animal safety studies with lovastatin revealed no toxicity of the type thought to be associated with compactin, clinical studies continued.
Large-scale trials confirmed the effectiveness of lovastatin. Observed tolerability continued to be excellent, and lovastatin was approved by the US FDA in 1987.[23] It was the first statin approved by the FDA.[24]
Lovastatin is also naturally produced by certain higher fungi, such as Pleurotus ostreatus (oyster mushroom) and closely related Pleurotus spp.[25] Research into the effect of oyster mushroom and its extracts on the cholesterol levels of laboratory animals has been extensive,[26][27][25][28][29][30][31][32][33][34][35][36] although the effect has been demonstrated in a very limited number of human subjects.[37]
In 1998, the FDA placed a ban on the sale of dietary supplements derived from red yeast rice, which naturally contains lovastatin, arguing that products containing prescription agents require drug approval.[38] Judge Dale A. Kimball of the United States District Court for the District of Utah, granted a motion by Cholestin's manufacturer, Pharmanex, that the agency's ban was illegal under the 1994 Dietary Supplement Health and Education Act because the product was marketed as a dietary supplement, not a drug.[39]

The objective is to decrease excess levels of cholesterol to an amount consistent with maintenance of normal body function. Cholesterol is biosynthesized in a series of more than 25 separate enzymatic reactions that initially involves three successive condensations of acetyl-CoA units to form the six-carbon compound 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA). This is reduced to mevalonate and then converted in a series of reactions to the isoprenes that are building-blocks of squalene, the immediate precursor to sterols, which cyclizes to lanosterol (a methylated sterol) and further metabolized to cholesterol. A number of early attempts to block the synthesis of cholesterol resulted in agents that inhibited late in the biosynthetic pathway between lanosterol and cholesterol. A major rate-limiting step in the pathway is at the level of the microsomal enzyme that catalyzes the conversion of HMG CoA to mevalonic acid, and that has been considered to be a prime target for pharmacologic intervention for several years.[15]
HMG CoA reductase occurs early in the biosynthetic pathway and is among the first committed steps to cholesterol formulation. Inhibition of this enzyme could lead to accumulation of HMG CoA, a water-soluble intermediate that is, then, capable of being readily metabolized to simpler molecules. This inhibition of reductase would lead to accumulation of lipophylic intermediates with a formal sterol ring.
Lovastatin was the first specific inhibitor of HMG CoA reductase to receive approval for the treatment of hypercholesterolemia. The first breakthrough in efforts to find a potent, specific, competitive inhibitor of HMG CoA reductase occurred in 1976, when Endo et al. reported the discovery of mevastatin, a highly functionalized fungal metabolite, isolated from cultures of Penicillium citrium.[40]
Biosynthesis


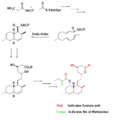
The biosynthesis of lovastatin occurs via an iterative type I polyketide synthase (PKS) pathway. The six genes that encode enzymes that are essential for the biosynthesis of lovastatin are lovB, lovC, lovA, lovD, lovG, and lovF .[41][42] The synthesis of dihydromonacolin L requires a total of 9-malonyl Coa .[41] It proceeds in the PKS pathway until it reaches (E) a hexaketide, where it undergoes a Diels-Alder cycloaddition to form the fused rings. After cyclization it continues through the PKS pathway until it reaches (I) a nonaketide, which then undergoes release from LovB through the thioesterase encoded by LovG. Dihydromonacolin L, (J), then undergoes oxidation and dehydration via a cytochrome P450 oxygenase encoded by LovA to obtain monacolin J, (L).
The MT domain from lovB is active in the conversion of (B) to (C) when it transfers a methyl group from S-adenosyl-L-methionine (SAM) to the tetraketide (C) .[41] Owing to the fact that LovB contains an inactive ER domain, LovC is required at specific steps to obtain fully reduced products. The domain organization of LovB, LovC, LovG and LovF is shown in Figure 2. The inactive ER domain of lovB is shown with an oval and where LovC acts in trans to LovB is shown with a red box.
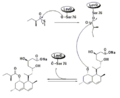
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF . Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
Total synthesis
A major bulk of work in the synthesis of lovastatin was done by M. Hirama in the 1980s.[43] [44] Hirama synthesized compactin and used one of the intermediates to follow a different path to get to lovastatin. The synthetic sequence is shown in the schemes below. The γ-lactone was synthesized using Yamada methodology starting with glutamic acid. Lactone opening was done using lithium methoxide in methanol and then silylation to give a separable mixture of the starting lactone and the silyl ether. The silyl ether on hydrogenolysis followed by Collins oxidation gave the aldehyde. Stereoselective preparation of (E,E)-diene was accomplished by addition of trans-crotyl phenyl sulfone anion, followed by quenching with Ac2O and subsequent reductive elimination of sulfone acetate. Condensation of this with lithium anion of dimethyl methylphosphonate gave compound 1. Compound 2 was synthesized as shown in the scheme in the synthetic procedure. Compounds 1 and 2 were then combined using 1.3 eq sodium hydride in THF followed by reflux in chlorobenzene for 82 hr under nitrogen to get the enone 3.
Simple organic reactions were used to get to lovastatin as shown in the scheme.
-
Cholesterol biosynthetic pathway
-
HMG CoA reductase reaction
-
Biosynthesis using Diels-Alder catalyzed cyclization
-
Biosynthesis using broadly specific acyltransferase
-
Synthesis of compounds 1 and 2
-
Complete lovastatin synthesis
Society and culture
Natural sources
Lovastatin is a naturally occurring compound found in low concentrations in food such as oyster mushrooms,[45] red yeast rice,[46] and Pu-erh.[47]
Brand names
Mevacor, Advicor (as a combination with niacin), Altocor, Altoprev
Other applications
In plant physiology, lovastatin has occasionally been used as inhibitor of cytokinin biosynthesis.[48]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin". Clinical Pharmacokinetics 47 (7): 463–474. 2008. doi:10.2165/00003088-200847070-00003. PMID 18563955.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Lovastatin Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/lovastatin.html.
- ↑ "Lovastatin Pregnancy and Breastfeeding Warnings". https://www.drugs.com/pregnancy/lovastatin.html.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 472. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA472.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Lovastatin - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Lovastatin.
- ↑ 8.0 8.1 "Lovastatin". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/Lovastatin.html.
- ↑ 9.0 9.1 "Mevacor, Altoprev (lovastatin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. http://reference.medscape.com/drug/mevacor-altoprev-lovastatin-342458#showall.
- ↑ 10.0 10.1 "Lovastatin". MedlinePlus. U.S. National Library of Medicine. 15 June 2012. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a688006.html.
- ↑ "Lovastatin". LactMed. U.S. National Library of Medicine. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~CSZXZ5:1.
- ↑ "Mevacor Side Effects Center". RxList. http://www.rxlist.com/mevacor-side-effects-drug-center.htm.
- ↑ "Grapefruit juice-drug interactions". British Journal of Clinical Pharmacology 46 (2): 101–110. August 1998. doi:10.1046/j.1365-2125.1998.00764.x. PMID 9723817.
- ↑ "Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid". Clinical Pharmacology and Therapeutics 63 (4): 397–402. April 1998. doi:10.1016/S0009-9236(98)90034-0. PMID 9585793.
- ↑ 15.0 15.1 "Discovery, biochemistry and biology of lovastatin". The American Journal of Cardiology 62 (15): 10J–15J. November 1988. doi:10.1016/0002-9149(88)90002-1. PMID 3055919.
- ↑ "Therapy insight: Potential of statins for cancer chemoprevention and therapy". Nature Clinical Practice. Oncology 2 (2): 82–89. February 2005. doi:10.1038/ncponc0097. PMID 16264880.
- ↑ "Statins as anti-cancer therapy; Can we translate preclinical and epidemiologic data into clinical benefit?". Discovery Medicine 20 (112): 413–427. December 2015. PMID 26760985.
- ↑ "Cell cycle-specific effects of lovastatin". Proceedings of the National Academy of Sciences of the United States of America 88 (9): 3628–3632. May 1991. doi:10.1073/pnas.88.9.3628. PMID 1673788. Bibcode: 1991PNAS...88.3628J.
- ↑ "Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase". Proceedings of the National Academy of Sciences of the United States of America 96 (14): 7797–7802. July 1999. doi:10.1073/pnas.96.14.7797. PMID 10393901. Bibcode: 1999PNAS...96.7797R.
- ↑ "Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains". Zeitschrift für Naturforschung C 58 (1–2): 62–64. Jan–Feb 2003. doi:10.1515/znc-2003-1-211. PMID 12622228.
- ↑ "Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen and oxygen by 13C NMR and mass spectrometry". J Am Chem Soc 107 (12): 3694–701. 1985. doi:10.1021/ja00298a046.
- ↑ "Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent". Proceedings of the National Academy of Sciences of the United States of America 77 (7): 3957–3961. July 1980. doi:10.1073/pnas.77.7.3957. PMID 6933445. Bibcode: 1980PNAS...77.3957A.
- ↑ FDA Orange Book Detail for application N019643 showing approval for 20 mg tablets on Aug 31, 1987 and 40 mg tablets on Dec 14, 1988
- ↑ "The origin of the statins. 2004". Atherosclerosis. Supplements 5 (3): 125–130. October 2004. doi:10.1016/j.atherosclerosissup.2004.08.033. PMID 15531285.
- ↑ 25.0 25.1 "Dose- and time-dependent hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats". Nutrition 14 (3): 282–286. March 1998. doi:10.1016/S0899-9007(97)00471-1. PMID 9583372.
- ↑ "Dietary mushroom (Pleurotus ostreatus) ameliorates atherogenic lipid in hypercholesterolaemic rats". Clinical and Experimental Pharmacology & Physiology 30 (7): 470–475. July 2003. doi:10.1046/j.1440-1681.2003.03857.x. PMID 12823261.
- ↑ "Hypocholesterolemic and antiatherogenic effect of oyster mushroom (Pleurotus ostreatus) in rabbits". Die Nahrung 43 (5): 339–342. October 1999. doi:10.1002/(SICI)1521-3803(19991001)43:5<339::AID-FOOD339>3.0.CO;2-5. PMID 10555301.
- ↑ "Evidence for the anti-hyperlipidaemic activity of the edible fungus Pleurotus ostreatus". British Journal of Biomedical Science 54 (4): 240–243. December 1997. PMID 9624732.
- ↑ "Role of oyster mushroom (Pleurotus florida) as hypocholesterolemic/antiatherogenic agent". Indian Journal of Experimental Biology 35 (10): 1070–1075. October 1997. PMID 9475042.
- ↑ "[Regulation of cholesterol metabolism with dietary addition of oyster mushrooms (Pleurotus ostreatus) in rats with hypercholesterolemia]" (in sk). Casopis Lekaru Ceskych 136 (6): 186–190. March 1997. PMID 9221192.
- ↑ "Effect of oyster mushroom (Pleurotus Ostreatus) and its ethanolic extract in diet on absorption and turnover of cholesterol in hypercholesterolemic rat". Die Nahrung 40 (4): 222–224. August 1996. doi:10.1002/food.19960400413. PMID 8810086.
- ↑ "Dietary oyster mushroom (Pleurotus ostreatus) accelerates plasma cholesterol turnover in hypercholesterolaemic rat". Physiological Research 44 (5): 287–291. 1995. PMID 8869262.
- ↑ "The effect of oyster mushroom (Pleurotus ostreatus), its ethanolic extract and extraction residues on cholesterol levels in serum, lipoproteins and liver of rat". Die Nahrung 39 (1): 98–99. 1995. doi:10.1002/food.19950390113. PMID 7898579.
- ↑ "Mechanism of hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats: reduction of cholesterol absorption and increase of plasma cholesterol removal". Zeitschrift für Ernährungswissenschaft 33 (1): 44–50. March 1994. doi:10.1007/BF01610577. PMID 8197787.
- ↑ "Effect of the oyster fungus on glycaemia and cholesterolaemia in rats with insulin-dependent diabetes". Physiological Research 42 (3): 175–179. 1993. PMID 8218150.
- ↑ "Cholesterol-lowering effect of the mushroom Pleurotus ostreatus in hereditary hypercholesterolemic rats". Annals of Nutrition & Metabolism 35 (4): 191–195. 1991. doi:10.1159/000177644. PMID 1897899.
- ↑ "Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects". Mymensingh Medical Journal 16 (1): 94–99. January 2007. doi:10.3329/mmj.v16i1.261. PMID 17344789.
- ↑ "FDA bans red yeast rice product". The Lancet 351 (9116): 1637. 1998. doi:10.1016/s0140-6736(05)77698-4.
- ↑ Cholesterol Treatment Upheld, The New York Times, 18 February 1999
- ↑ "ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium". The Journal of Antibiotics 29 (12): 1346–1348. December 1976. doi:10.7164/antibiotics.29.1346. PMID 1010803.
- ↑ 41.0 41.1 41.2 "Biosynthesis of lovastatin and related metabolites formed by fungal iterative PKS enzymes". Biopolymers 93 (9): 755–763. September 2010. doi:10.1002/bip.21428. PMID 20577995.
- ↑ "LovG: the thioesterase required for dihydromonacolin L release and lovastatin nonaketide synthase turnover in lovastatin biosynthesis". Angewandte Chemie 52 (25): 6472–6475. June 2013. doi:10.1002/anie.201302406. PMID 23653178.
- ↑ "A chiral total synthesis of compactin". J. Am. Chem. Soc. 104 (15): 4251–4253. 1982. doi:10.1021/ja00379a037.
- ↑ "Synthesis of (+)-Mevinolin starting from Naturally occurring building blocks and using an asymmetry inducing reaction". Tetrahedron Lett. 24 (17): 1811–1812. 1983. doi:10.1016/S0040-4039(00)81777-3.
- ↑ "Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase-lovastatin". Experimental Mycology 19 (1): 1–6. March 1995. doi:10.1006/emyc.1995.1001. PMID 7614366.
- ↑ "Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials". Chinese Medicine 1 (1): 4. November 2006. doi:10.1186/1749-8546-1-4. PMID 17302963.
- ↑ "Exposure assessment of lovastatin in Pu-erh tea". International Journal of Food Microbiology 164 (1): 26–31. June 2013. doi:10.1016/j.ijfoodmicro.2013.03.018. PMID 23587710.
- ↑ "Assessment of lovastatin application as tool in probing cytokinin-mediated cell cycle regulation". Physiologia Plantarum 125 (2): 260–267. 2005. doi:10.1111/j.1399-3054.2005.00556.x.
External links
- "Lovastatin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/lovastatin.
 |