Biology:PDK2
 Generic protein structure example |
Pyruvate dehydrogenase kinase isoform 2 (PDK2) also known as pyruvate dehydrogenase lipoamide kinase isozyme 2, mitochondrial is an enzyme that in humans is encoded by the PDK2 gene.[1][2] PDK2 is an isozyme of pyruvate dehydrogenase kinase.
Structure
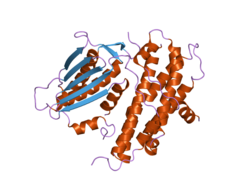
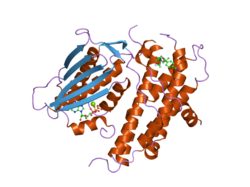
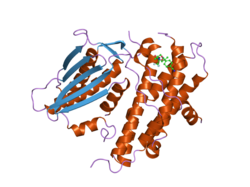
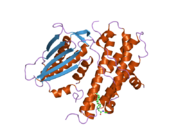
The protein encoded by the PDK2 gene has two sites, an active site and an allosteric site that allow for the activity and regulation of this enzyme. There are many structural motifs that are important to the regulation of this enzyme. Nov3r and AZ12 inhibitors bind at the lipoamide binding site that is located at one end of the R domain. Pfz3 binds in an extended site at the other end of the R domain. One inhibitor, dicholoroacetate (DCA), binds at the center of the R domain.[3] Within the active site, there are three amino acid residues, R250, T302, and Y320, that make the kinase resistant to the inhibitor dichloroacetate, which uncouples the active site from the allosteric site. This supports the theory that R250, T302, and Y320 stabilize the "open" and "closed" conformations of the built-in lid that controls the access of a nucleotide into the nucleotide-binding cavity. This strongly suggests that the mobility of ATP lid is central to the allosteric regulation of PDHK2 activity serving as a conformational switch required for communication between the active site and allosteric sites in the kinase molecule.[4] There is also a DW-motif that is crucial in mediating DCA, nucleotide, and lipoyl domain binding site communication. This network is responsible for rendering PDK2 locked in the closed, or inactive conformation.[5]
Function
The Pyruvate Dehydrogenase (PDH) complex must be tightly regulated due to its central role in general metabolism. Within the complex, there are three serine residues on the E1 component that are sites for phosphorylation; this phosphorylation inactivates the complex. In humans, there have been four isozymes of Pyruvate Dehydrogenase Kinase that have been shown to phosphorylate these three sites: PDK1, PDK2, PDK3, and PDK4.[6] PDK2 has been identified as the most abundant isoform in human tissues. Through many studies, it has been made clear that the activity of this enzyme is essential, even at rest, to regulate glycolysis/carbodydrate oxidation and producing metabolites for oxidative phosphorylation and the electron transport chain. These studies have illustrated that the kinetics of the PDK isoform population, specifically PDK2, is more important in determining PDH activity than measuring PDK activity.[7]
Regulation
As the primary regulators of a crucial step in the central metabolic pathway, the pyruvate dehydrogenase family is tightly regulated itself by a myriad of factors. PDK2 activity is modulated by low levels of hydrogen peroxide; this happens because the compound temporarily oxidizes the cysteine residues 45 and 392 on the enzyme, resulting in an inactive PDK2 and greater PDH activity. These conditions also inactivate the TCA cycle, the next step in aerobic respiration. This alludes to the fact that when there is a high level of O2 production in the mitochondria, which may occur because of nutrient excess, the increase in the products serve as a negative feedback that control mitochondria metabolism.[8] PDK2, in conjunction with PDK3 and PDK4, are primary targets of Peroxisome proliferator-activated receptor delta or beta, with PDK2 having two elements that respond to these receptors.[9]
Clinical significance
All of the pyruvate dehydrogenase isozymes have been associated with various metabolic disorders, including diabetes. This is due to a mechanism by which consistently elevated free fatty acid levels stimulate the PDK enzymes, particularly, PDK2 and PDK4 in the liver. In stimulating this activity, there is less PDH activity, and therefore less glucose uptake.[10]
Cancer
As the PDK enzymes are associated with central metabolism and growth, they are often associated with various mechanisms of cancer progression. Enhanced PDK2 activity leads to increased glycolysis and lactic acid production, known as the Warburg effect. In some studies, the wild-type form of tumor protein p53 prevents manifestation of tumorigenesis by regulating PDK2 activity.[11] Additionally, inhibition of PDK2 subsequently inhibits HIF1A in cancer cells by both a prolyl-hydroxylase (PHD)-dependent mechanism and a PHD-independent mechanism. Therefore, mitochondria-targeting metabolic modulators increase pyruvate dehydrogenase activity, and suppress angiogenesis as well, normalizing the pseudo-hypoxic signals that lead to normoxic HIF1A activation in solid tumors.[12]
References
- ↑ "Diversity of the pyruvate dehydrogenase kinase gene family in humans". The Journal of Biological Chemistry 270 (48): 28989–94. Dec 1995. doi:10.1074/jbc.270.48.28989. PMID 7499431.
- ↑ "Entrez Gene: PDK2 pyruvate dehydrogenase kinase, isozyme 2". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5164.
- ↑ "Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands". Biochemistry 45 (2): 402–15. Jan 2006. doi:10.1021/bi051402s. PMID 16401071.
- ↑ "Allosteric coupling in pyruvate dehydrogenase kinase 2". Biochemistry 47 (32): 8358–66. Aug 2008. doi:10.1021/bi800631h. PMID 18627174.
- ↑ "Pivotal role of the C-terminal DW-motif in mediating inhibition of pyruvate dehydrogenase kinase 2 by dichloroacetate". The Journal of Biological Chemistry 284 (49): 34458–67. Dec 2009. doi:10.1074/jbc.M109.065557. PMID 19833728.
- ↑ "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal 358 (Pt 1): 69–77. Aug 2001. doi:10.1042/0264-6021:3580069. PMID 11485553.
- ↑ "PDH activation during in vitro muscle contractions in PDH kinase 2 knockout mice: effect of PDH kinase 1 compensation". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 300 (6): R1487-93. Jun 2011. doi:10.1152/ajpregu.00498.2010. PMID 21411764.
- ↑ Hurd, TR; Collins, Y; Abakumova, I; Chouchani, ET; Baranowski, B; Fearnley, IM; Prime, TA; Murphy, MP et al. (12 October 2012). "Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species.". The Journal of Biological Chemistry 287 (42): 35153–60. doi:10.1074/jbc.m112.400002. PMID 22910903.
- ↑ Degenhardt, T; Saramäki, A; Malinen, M; Rieck, M; Väisänen, S; Huotari, A; Herzig, KH; Müller, R et al. (14 September 2007). "Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta.". Journal of Molecular Biology 372 (2): 341–55. doi:10.1016/j.jmb.2007.06.091. PMID 17669420.
- ↑ Bajotto, G; Murakami, T; Nagasaki, M; Qin, B; Matsuo, Y; Maeda, K; Ohashi, M; Oshida, Y et al. (March 2006). "Increased expression of hepatic pyruvate dehydrogenase kinases 2 and 4 in young and middle-aged Otsuka Long-Evans Tokushima Fatty rats: induction by elevated levels of free fatty acids.". Metabolism: Clinical and Experimental 55 (3): 317–23. doi:10.1016/j.metabol.2005.09.014. PMID 16483874.
- ↑ Contractor, T; Harris, CR (15 January 2012). "p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2.". Cancer Research 72 (2): 560–7. doi:10.1158/0008-5472.can-11-1215. PMID 22123926.
- ↑ Sutendra, G; Dromparis, P; Kinnaird, A; Stenson, TH; Haromy, A; Parker, JM; McMurtry, MS; Michelakis, ED (28 March 2013). "Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer.". Oncogene 32 (13): 1638–50. doi:10.1038/onc.2012.198. PMID 22614004.
Further reading
- "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". American Journal of Physiology. Endocrinology and Metabolism 284 (5): E855–62. May 2003. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- "Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2". The Biochemical Journal 339 (2): 319–28. Apr 1999. doi:10.1042/0264-6021:3390319. PMID 10191262.
- "The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase". Journal of Immunology 163 (4): 1894–905. Aug 1999. doi:10.4049/jimmunol.163.4.1894. PMID 10438924.
- "Marked differences between two isoforms of human pyruvate dehydrogenase kinase". The Journal of Biological Chemistry 275 (21): 15773–81. May 2000. doi:10.1074/jbc.M909488199. PMID 10748134.
- "Structure of pyruvate dehydrogenase kinase. Novel folding pattern for a serine protein kinase". The Journal of Biological Chemistry 276 (40): 37443–50. Oct 2001. doi:10.1074/jbc.M104285200. PMID 11483605.
- "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal 358 (Pt 1): 69–77. Aug 2001. doi:10.1042/0264-6021:3580069. PMID 11485553.
- "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". The Journal of Biological Chemistry 276 (40): 37223–9. Oct 2001. doi:10.1074/jbc.M103069200. PMID 11486000.
- "Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet". American Journal of Physiology. Endocrinology and Metabolism 281 (6): E1151–8. Dec 2001. doi:10.1152/ajpendo.2001.281.6.e1151. PMID 11701428.
- "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". The Biochemical Journal 366 (Pt 1): 129–36. Aug 2002. doi:10.1042/BJ20020301. PMID 11978179.
- "Formation of functional heterodimers by isozymes 1 and 2 of pyruvate dehydrogenase kinase". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1645 (2): 183–92. Feb 2003. doi:10.1016/S1570-9639(02)00542-3. PMID 12573248.
- "Facilitated interaction between the pyruvate dehydrogenase kinase isoform 2 and the dihydrolipoyl acetyltransferase". The Journal of Biological Chemistry 278 (36): 33681–93. Sep 2003. doi:10.1074/jbc.M212733200. PMID 12816949.
- "Rapid upregulation of pyruvate dehydrogenase kinase activity in human skeletal muscle during prolonged exercise". Journal of Applied Physiology 97 (4): 1261–7. Oct 2004. doi:10.1152/japplphysiol.00132.2004. PMID 15169745.
- "Pyruvate dehydrogenase kinase isoform 2 activity limited and further inhibited by slowing down the rate of dissociation of ADP". Biochemistry 43 (42): 13432–41. Oct 2004. doi:10.1021/bi049488x. PMID 15491150.
- "Pyruvate dehydrogenase kinase isoform 2 activity stimulated by speeding up the rate of dissociation of ADP". Biochemistry 43 (42): 13442–51. Oct 2004. doi:10.1021/bi0494875. PMID 15491151.
- "Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells". The FEBS Journal 272 (12): 3004–14. Jun 2005. doi:10.1111/j.1742-4658.2005.04713.x. PMID 15955060.
 |