Biology:Pyruvate dehydrogenase kinase
| Pyruvate dehydrogenase kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
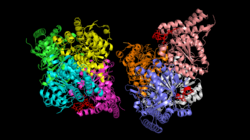 The areas around the three phosphorylation sites are shown in red. Site 1 is in the bottom left corner, site 2 in the top right, and site 3 in the bottom right. | |||||||||
| Identifiers | |||||||||
| EC number | 2.7.11.2 | ||||||||
| CAS number | 2620256 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Pyruvate dehydrogenase kinase (also pyruvate dehydrogenase complex kinase, PDC kinase, or PDK; EC 2.7.11.2) is a kinase enzyme which acts to inactivate the enzyme pyruvate dehydrogenase by phosphorylating it using ATP.
PDK thus participates in the regulation of the pyruvate dehydrogenase complex of which pyruvate dehydrogenase is the first component. Both PDK and the pyruvate dehydrogenase complex are located in the mitochondrial matrix of eukaryotes. The complex acts to convert pyruvate (a product of glycolysis in the cytosol) to acetyl-coA, which is then oxidized in the mitochondria to produce energy, in the citric acid cycle. By downregulating the activity of this complex, PDK will decrease the oxidation of pyruvate in mitochondria and increase the conversion of pyruvate to lactate in the cytosol.
The opposite action of PDK, namely the dephosphorylation and activation of pyruvate dehydrogenase, is catalyzed by a phosphoprotein phosphatase called pyruvate dehydrogenase phosphatase.
(Pyruvate dehydrogenase kinase should not be confused with Phosphoinositide-dependent kinase-1, which is also sometimes known as "PDK1".)
Phosphorylation sites
PDK can phosphorylate a serine residue on pyruvate dehydrogenase at three possible sites. Some evidence has shown that phosphorylation at site 1 will nearly completely deactivate the enzyme while phosphorylation at sites 2 and 3 had only a small contribution to complex inactivation.[1] Therefore, it is phosphorylation at site 1 that is responsible for pyruvate dehydrogenase deactivation.
Isozymes
There are four known isozymes of PDK in humans:
The primary sequencing between the four isozymes are conserved with 70% identity. The greatest differences occur near the N-terminus.[2]
PDK1 is the largest of the four with 436 residues while PDK2, PDK3 and PDK4 have 407, 406, and 411 residues respectively. The isozymes have different activity and phosphorylation rates at each site. At site 1 in order from fastest to slowest, PDK2 > PDK4 ≈ PDK1 > PDK3. For site 2, PDK3 > PDK4 > PDK2 > PDK1. Only PDK1 can phosphorylate site 3. However, it has been shown that these activities are sensitive to slight changes in pH so the microenvironment of the PDK isozymes may change the reaction rates.[3][4]
Isozyme abundance has also been shown to be tissue specific. PDK1 is ample in heart cells. PDK3 is most abundant in testis. PDK2 is present in most tissues but low in spleen and lung cells. PDK4 is predominantly found in skeletal muscle and heart tissues.[5]
Mechanism
Pyruvate dehydrogenase is deactivated when phosphorylated by PDK. Normally, the active site of pyruvate dehydrogenase is in a stabilized and ordered conformation supported by a network of hydrogen bonds. However, phosphorylation by PDK at site 1 causes steric clashes with another nearby serine residue due to both the increased size and negative charges associated with the phosphorylated residue.[6] This disrupts the hydrogen bond network and disorders the conformation of two phosphorylation loops. These loops prevent the reductive acetylation step, thus halting overall activity of the enzyme.[7] The conformational changes and mechanism of deactivation for phosphorylation at sites 2 and 3 are not known at this time.
Regulation

Pyruvate dehydrogenase kinase is activated by ATP, NADH and acetyl-CoA. It is inhibited by ADP, NAD+, CoA-SH and pyruvate.[9]
Each isozyme responds to each of these factors slightly differently. NADH stimulates PDK1 activity by 20% and PDK2 activity by 30%. NADH with acetyl-CoA increases activity in these enzymes by 200% and 300% respectively. In similar conditions, PDK3 is unresponsive to NADH and inhibited by NADH with acetyl-CoA. PDK4 has a 200% activity increase with NADH, but adding acetyl-CoA does not increase activity further.[5]
Disease relevance
PDK isoforms are elevated in obesity, diabetes, heart failure, and cancer.[10] Some studies have shown that cells that lack insulin (or are insensitive to insulin) overexpress PDK4.[11] As a result, the pyruvate formed from glycolysis cannot be oxidized which leads to hyperglycaemia due to the fact that glucose in the blood cannot be used efficiently. Therefore, several drugs target PDK4 hoping to treat type II diabetes.[12]
PDK1 has shown to have increased activity in hypoxic cancer cells due to the presence of HIF-1. PDK1 shunts pyruvate away from the citric acid cycle and keeps the hypoxic cell alive.[13] Therefore, PDK1 inhibition has been suggested as an antitumor therapy since PDK1 prevents apoptosis in these cancerous cells.[14] Similarly, PDK3 has been shown to be overexpressed in colon cancer cell lines.[15] Three proposed inhibitors are AZD7545 and dichloroacetate which both bind to PDK1, and Radicicol which binds to PDK3.[16]
Mutations in the PDK3 gene are a rare cause of X-linked Charcot-Marie-Tooth disease (CMTX6).[17][18]
In dogs, specifically Doberman Pinschers, a mutation in the PDK4 gene is associated with dilated cardiomyopathy (DCM).[19][20][21]
References
- ↑ "Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart". Biochemistry 17 (12): 2364–70. June 1978. doi:10.1021/bi00605a017. PMID 678513.
- ↑ "Molecular cloning of the p45 subunit of pyruvate dehydrogenase kinase". The Journal of Biological Chemistry 269 (47): 29720–4. November 1994. doi:10.1016/S0021-9258(18)43940-3. PMID 7961963. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=7961963.
- ↑ "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". The Journal of Biological Chemistry 276 (40): 37223–9. October 2001. doi:10.1074/jbc.M103069200. PMID 11486000.
- ↑ "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal 358 (Pt 1): 69–77. August 2001. doi:10.1042/0264-6021:3580069. PMID 11485553.
- ↑ 5.0 5.1 "Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex". The Biochemical Journal 329 (1): 191–6. January 1998. doi:10.1042/bj3290191. PMID 9405293.
- ↑ "Probing the mechanism of inactivation of human pyruvate dehydrogenase by phosphorylation of three sites". The Journal of Biological Chemistry 276 (8): 5731–8. February 2001. doi:10.1074/jbc.M007558200. PMID 11092882.
- ↑ "Structural basis for inactivation of the human pyruvate dehydrogenase complex by phosphorylation: role of disordered phosphorylation loops". Structure 16 (12): 1849–59. December 2008. doi:10.1016/j.str.2008.10.010. PMID 19081061.
- ↑ "Monovalent cation requirement for ADP inhibition of pyruvate dehydrogenase kinase". Biochemical and Biophysical Research Communications 59 (4): 1341–8. August 1974. doi:10.1016/0006-291X(74)90461-6. PMID 4370205.
- ↑ "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". American Journal of Physiology. Endocrinology and Metabolism 284 (5): E855-62. May 2003. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- ↑ "Role of the Pyruvate Dehydrogenase Complex in Metabolic Remodeling: Differential Pyruvate Dehydrogenase Complex Functions in Metabolism". Diabetes Medical Journal 42 (4): 270–281. 2018. doi:10.4093/dmj.2018.0101. PMID 30136450.
- ↑ "Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects". Molecular Genetics and Metabolism 65 (2): 181–6. October 1998. doi:10.1006/mgme.1998.2748. PMID 9787110. https://zenodo.org/record/1229928.
- ↑ "Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation". Biochemical Society Transactions 31 (Pt 6): 1143–51. December 2003. doi:10.1042/bst0311143. PMID 14641014.
- ↑ "HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia". Cell Metabolism 3 (3): 177–85. March 2006. doi:10.1016/j.cmet.2006.02.002. PMID 16517405.
- ↑ "A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth". Cancer Cell 11 (1): 37–51. January 2007. doi:10.1016/j.ccr.2006.10.020. PMID 17222789.
- ↑ "Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer". The American Journal of Pathology 179 (3): 1405–14. September 2011. doi:10.1016/j.ajpath.2011.05.050. PMID 21763680.
- ↑ "Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol". Structure 15 (8): 992–1004. August 2007. doi:10.1016/j.str.2007.07.001. PMID 17683942.
- ↑ Online Mendelian Inheritance in Man (OMIM) Charcot-Marie-tooth disease, X-linked dominant, 6; CMTX6 -300905
- ↑ "A new locus for X-linked dominant Charcot-Marie-Tooth disease (CMTX6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (PDK3) gene". Human Molecular Genetics 22 (7): 1404–16. April 2013. doi:10.1093/hmg/dds557. PMID 23297365.
- ↑ "Functional Consequences of PDK4 Deficiency in Doberman Pinscher Fibroblasts". Scientific Reports 10 (1): 3930. March 2020. doi:10.1038/s41598-020-60879-6. PMID 32127618. Bibcode: 2020NatSR..10.3930B.
- ↑ "PDK4 Deficiency Induces Intrinsic Apoptosis in Response to Starvation in Fibroblasts from Doberman Pinschers with Dilated Cardiomyopathy". BioResearch Open Access 6 (1): 182–191. 2017. doi:10.1089/biores.2017.0023. PMID 29285418.
- ↑ "A splice site mutation in a gene encoding for PDK4, a mitochondrial protein, is associated with the development of dilated cardiomyopathy in the Doberman pinscher". Human Genetics 131 (8): 1319–25. August 2012. doi:10.1007/s00439-012-1158-2. PMID 22447147.
External links
- pyruvate+dehydrogenase+kinase at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 2.7.11.2
 |

