Biology:Qrr RNA
| Quorum regulatory RNA | |
|---|---|
 | |
| Identifiers | |
| Symbol | Qrr |
| Alt. Symbols | Qrr1–5 |
| Rfam | RF00378 |
| Other data | |
| RNA type | sRNA |
| Domain(s) | Vibrio |
| PDB structures | PDBe |
Introduction
Qrr (Quorum regulatory RNA)[1] is a small non-coding RNA that is thought to be involved in the regulation of quorum sensing in Vibrio species. The use of small RNAs for vital functions like metabolism, infection cycling, and stress response is ubiquitous among bacteria.[2] Qrr operates as part of a negative feedback loop which regulates the shift in cell state from that of low density populations to that in high density populations.[3] This feedback system allows for rapid responses to changes in population cell density, eliminating the production of energy-costly molecules.[4] It is believed that these RNAs, guided by a protein, Hfq, can mediate the destabilization of the quorum-sensing master regulators LuxR/HapR/VanT mRNAs.[2][5] This group of non-coding RNAs are trans-acting small RNAs (sRNAs) that bind via base pairing to the untranscribed domain of their mRNA targets. This binding results in degradation or stabilization, deciding their translational fate.[6]
Qrr RNA Characteristics
Genes, Expression, and Mechanism
There are 5 different qrr genes (Qrr1–5) in V. harveyi; of these, qrr2, 3 and 4 are activated by LuxR.[5] Other Vibrio species contain varying number of these genes, with overlapping functions and promotion.[6] Each of these Qrr RNAs are expressed at different times, fluctuating in level.[7] Each gene is expressed individually based on growth conditions, with unique factors and regulators controlling their respective expression.[6] For example, LuxT transcriptionally represses qrr1, but does not regulate the other qrr genes.[8] The genes are expressed in this order from lowest to highest: Qrr5, Qrr1, Qrr3, Qrr2, Qrr4.[8]
Exactly 20 mRNA targets of the RNAs have been established in Vibrio.[7] Four regulation strategies are utilized by these molecules through unique base-pairing interactions with mRNA targets: sequestration for luxO, coupled degradation for luxM, uncovering the RBS of aphA, and catalytic degradation for luxR.[7] Each Qrr RNA contains specific binding regions to differentiate between different mRNA targets.[9] Translation of AphA is enhanced for low cell density conditions, whereas LuxR is inhibited for high cell density conditions.[6] Qrr2 was found to be unique in possessing two promoters and utilized by other species in addition to Vibrio.[6] The unique type of regulation by Qrr RNA likely produces expression patterns that protein transcription factors cannot.[4]
The protein Hfq serves as a mediator between each qrr RNA and their respective mRNA targets.[7] It also protects the unstable molecules from free degradation by RNase. Abundance of Hfq limits qrr RNA binding, as the separate RNAs compete for its safeguarding behavior.[7]
Structure and Evolution
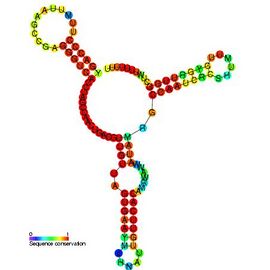
Qrr RNAs were first identified in 2004 in bioinformatic screenings of several Vibrio species.[1] It is believed that the stem loop portion of the RNA structure was integral to its primordial functions, with other functionalities resulting from sequence mutations.[10] The molecule is composed of four stem loops (loops seen in "Quorum regulatory RNA" image): two stem loops operate by base-pairing to the mRNA targets, the second also insulates the structure from Rnase E-mediated degradation, the third assists in stabilizing base-pairing, and the fourth is utilized as a terminator.[4]
The qrr genes share 80% sequence similarity, with predictions of analogous secondary structures.[2] In the event of deficiency in a single Qrr RNA, the other genes are upregulated to compensate for the loss, but can also have independent functions.[2] Two known feedback loops account for the expression adjustment: HapR-Qrr and LuxO-Qrr feedback loops.[2] This functional duality give plasticity to bacteria possessing these genes, allowing them to react accordingly to environmental and communal conditions.[8]
Applications and Examples
Quorum Sensing for Bioluminescence in V. harveyi
Detailed mechanistic pathways have been uncovered for how Qrr RNA is utilized in V. harveyi for the phenomenon of bioluminescence. Three autoinducers (AIs) are produced by this species: AI-1, LuxS, and CAI-1.[8] LuxN, LuxPq, and CqsS recognize these AIs, respectively. Few AIs are produced when cell density is low, which leads to a phosphorylated LuxO, along with sigma factor 54, activating qrr1-5 expression.[8] Binding sites for these two regulators are upstream of each qrr.[8] Post-transcriptionally, the Qrr RNAs promote the expression of low cell density master regulator aphA and represses expression of high cell density master regulator luxR. Their expression also inhibitors expression of the luciferase operon, which allows luminescent output for V. harveyi.[8] The opposite phenomenon occurs in high cell density, with high AI expression and subsequent reversal of aphA and luxR expression levels. The luciferase operon is expressed and luminescence occurs for cell communication.[8] The phosphorylation of LuxO is key to this mechanism, not necessarily luxO expression.[8]
Other Functions
Novel qrr RNAs have also been investigated recently in species outside of the Vibrio genus. One such RNA, AmiL, was identified in Pseudomonas aeruginosa.[11] AmiL was found to be involved in virulence of P. aeruginosa, including mammalian cytotoxicity, biofilm formation, and motility.[11] This RNA plays into a larger network of quorum sensing which has yet to be elucidated.
An additional 16 Qrr RNA targets outside of quorum sensing regulatory networks have been identified.[4] Among these are certain quorum-sensing-controlled virulence factors and chemotaxis receptors, thought previously to only be regulated by protein transcription factors. Since the production of these factors taxes the cell, the rapid response regulation provided by Qrr RNA could be advantageous in energy-conserving repression.[4]
References
- ↑ 1.0 1.1 Lenz, DH; Mok KC; Lilley BN; Kulkarni RV; Wingreen NS; Bassler BL (2004). "The Small RNA Chaperone Hfq and Multiple Small RNAs Control Quorum Sensing in Vibrio harveyi and Vibrio cholerae". Cell 118 (1): 69–82. doi:10.1016/j.cell.2004.06.009. PMID 15242645.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing". EMBO J 28 (4): 429–439. 2009. doi:10.1038/emboj.2008.300. PMID 19165149.
- ↑ "Negative Feedback Loops Involving Small Regulatory RNAs Precisely Control the Vibrio harveyi Quorum-Sensing Response". Mol Cell 37 (4): 567–579. 2010. doi:10.1016/j.molcel.2010.01.022. PMID 20188674.
- ↑ 4.0 4.1 4.2 4.3 4.4 Shao, Yi; Feng, Lihui; Rutherford, Steven T; Papenfort, Kai; Bassler, Bonnie L (2013-07-09). "Functional determinants of the quorum-sensing non-coding RNAs and their roles in target regulation". The EMBO Journal 32 (15): 2158–2171. doi:10.1038/emboj.2013.155. ISSN 0261-4189. PMID 23838640.
- ↑ 5.0 5.1 Tu, K.; Waters, C.; Svenningsen, S.; Bassler, B. (Nov 2008). "A small-RNA-mediated negative feedback loop controls quorum-sensing dynamics in Vibrio harveyi". Molecular Microbiology 70 (4): 896–907. doi:10.1111/j.1365-2958.2008.06452.x. ISSN 0950-382X. PMID 18808382.
- ↑ 6.0 6.1 6.2 6.3 6.4 Tague, J. G.; Hong, J.; Kalburge, S. S.; Boyd, E. F. (2022-01-18). O’Toole, George. ed. "Regulatory Small RNA Qrr2 Is Expressed Independently of Sigma Factor-54 and Can Function as the Sole Qrr Small RNA To Control Quorum Sensing in Vibrio parahaemolyticus" (in en). Journal of Bacteriology 204 (1): e00350–21. doi:10.1128/JB.00350-21. ISSN 0021-9193. PMID 34633869.
- ↑ 7.0 7.1 7.2 7.3 7.4 Feng, Lihui; Rutherford, Steven T.; Papenfort, Kai; Bagert, John D.; van Kessel, Julia C.; Tirrell, David A.; Wingreen, Ned S.; Bassler, Bonnie L. (2015-01-15). "A Qrr Noncoding RNA Deploys Four Different Regulatory Mechanisms to Optimize Quorum-Sensing Dynamics" (in en). Cell 160 (1): 228–240. doi:10.1016/j.cell.2014.11.051. ISSN 0092-8674. PMID 25579683.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 Eickhoff, Michaela J.; Fei, Chenyi; Huang, Xiuliang; Bassler, Bonnie L. (2021-04-01). "LuxT controls specific quorum-sensing-regulated behaviors in Vibrionaceae spp. via repression of qrr1, encoding a small regulatory RNA" (in en). PLOS Genetics 17 (4): e1009336. doi:10.1371/journal.pgen.1009336. ISSN 1553-7404. PMID 33793568.
- ↑ Shao, Yi; Bassler, Bonnie L. (February 2012). "Quorum-sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets". Molecular Microbiology 83 (3): 599–611. doi:10.1111/j.1365-2958.2011.07959.x. ISSN 1365-2958. PMID 22229925.
- ↑ Dutcher, H. Auguste; Raghavan, Rahul (2018-04-06). Storz, Gisela; Papenfort, Kai. eds. "Origin, Evolution, and Loss of Bacterial Small RNAs" (in en). Microbiology Spectrum 6 (2): 6.2.12. doi:10.1128/microbiolspec.RWR-0004-2017. ISSN 2165-0497. PMID 29623872.
- ↑ 11.0 11.1 Pu, Jieying; Zhang, Shebin; He, Xi; Zeng, Jianming; Shen, Cong; Luo, Yanfen; Li, Honglin; Long, Yifei et al. (2022-04-27). Rather, Philip N.. ed. "The Small RNA AmiL Regulates Quorum Sensing-Mediated Virulence in Pseudomonas aeruginosa PAO1" (in en). Microbiology Spectrum 10 (2): e02211–21. doi:10.1128/spectrum.02211-21. ISSN 2165-0497. PMID 35262393.
Further reading
- Shao, Y; Bassler, BL (Apr 3, 2014). "Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae.". Molecular Microbiology 92 (5): 921–930. doi:10.1111/mmi.12599. PMID 24698180.
- Tu, Kimberly C. (January 15, 2007). "Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi". Genes Dev. 21 (2): 221–233. doi:10.1101/gad.1502407. PMID 17234887.
- Waters, Christopher M. (October 1, 2006). "The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers". Genes Dev 20 (19): 2754–67. doi:10.1101/gad.1466506. PMID 17015436. PMC 1578700. https://pubmed.ncbi.nlm.nih.gov/17015436/.
External links
 |