Biology:Non-coding RNA
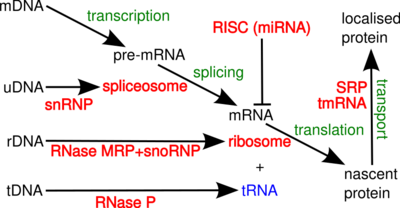
A non-coding RNA (ncRNA) is a functional RNA molecule that is not translated into a protein. The DNA sequence from which a functional non-coding RNA is transcribed is often called an RNA gene. Abundant and functionally important types of non-coding RNAs include transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), as well as small RNAs such as microRNAs, siRNAs, piRNAs, snoRNAs, snRNAs, exRNAs, scaRNAs and the long ncRNAs such as Xist and HOTAIR.
The number of non-coding RNAs within the human genome is unknown; however, recent transcriptomic and bioinformatic studies suggest that there are thousands of non-coding transcripts.[1][2][3][4][5][6][7] Many of the newly identified ncRNAs have not been validated for their function.[8] There is no consensus in the literature on how much of non-coding transcription is functional. Some researchers have argued that many ncRNAs are non-functional (sometimes referred to as "junk RNA"), spurious transcriptions.[9][10] Others, however, disagree, arguing instead that many non-coding transcripts do have functions and that those functions are being and will continue to be discovered.[11][12]
History and discovery
Nucleic acids were first discovered in 1868 by Friedrich Miescher,[13] and by 1939, RNA had been implicated in protein synthesis.[14] Two decades later, Francis Crick predicted a functional RNA component which mediated translation; he reasoned that RNA is better suited to base-pair with an mRNA transcript than a pure polypeptide.[15]
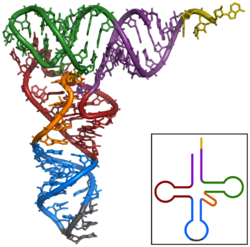
The first non-coding RNA to be characterised was an alanine tRNA found in baker's yeast, its structure was published in 1965.[16] To produce a purified alanine tRNA sample, Robert W. Holley et al. used 140kg of commercial baker's yeast to give just 1g of purified tRNAAla for analysis.[17] The 80 nucleotide tRNA was sequenced by first being digested with Pancreatic ribonuclease (producing fragments ending in Cytosine or Uridine) and then with takadiastase ribonuclease Tl (producing fragments which finished with Guanosine). Chromatography and identification of the 5' and 3' ends then helped arrange the fragments to establish the RNA sequence.[17] Of the three structures originally proposed for this tRNA,[16] the 'cloverleaf' structure was independently proposed in several following publications.[18][19][20][21] The cloverleaf secondary structure was finalised following X-ray crystallography analysis performed by two independent research groups in 1974.[22][23]
Ribosomal RNA was next to be discovered, followed by URNA in the early 1980s. Since then, the discovery of new non-coding RNAs has continued with snoRNAs, Xist, CRISPR and many more.[24] Recent notable additions include riboswitches and miRNA; the discovery of the RNAi mechanism associated with the latter earned Craig C. Mello and Andrew Fire the 2006 Nobel Prize in Physiology or Medicine.[25]
Recent discoveries of ncRNAs have been achieved through both experimental and bioinformatic methods.
Biological roles
Noncoding RNAs belong to several groups and are involved in many cellular processes.[26] These range from ncRNAs of central importance that are conserved across all or most cellular life through to more transient ncRNAs specific to one or a few closely related species. The more conserved ncRNAs are thought to be molecular fossils or relics from the last universal common ancestor and the RNA world, and their current roles remain mostly in regulation of information flow from DNA to protein.[27][28][29]
In translation

Many of the conserved, essential and abundant ncRNAs are involved in translation. Ribonucleoprotein (RNP) particles called ribosomes are the 'factories' where translation takes place in the cell. The ribosome consists of more than 60% ribosomal RNA; these are made up of 3 ncRNAs in prokaryotes and 4 ncRNAs in eukaryotes. Ribosomal RNAs catalyse the translation of nucleotide sequences to protein. Another set of ncRNAs, Transfer RNAs, form an 'adaptor molecule' between mRNA and protein. The H/ACA box and C/D box snoRNAs are ncRNAs found in archaea and eukaryotes. RNase MRP is restricted to eukaryotes. Both groups of ncRNA are involved in the maturation of rRNA. The snoRNAs guide covalent modifications of rRNA, tRNA and snRNAs; RNase MRP cleaves the internal transcribed spacer 1 between 18S and 5.8S rRNAs. The ubiquitous ncRNA, RNase P, is an evolutionary relative of RNase MRP.[31] RNase P matures tRNA sequences by generating mature 5'-ends of tRNAs through cleaving the 5'-leader elements of precursor-tRNAs. Another ubiquitous RNP called SRP recognizes and transports specific nascent proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes. In bacteria, Transfer-messenger RNA (tmRNA) is an RNP involved in rescuing stalled ribosomes, tagging incomplete polypeptides and promoting the degradation of aberrant mRNA.[citation needed]
In RNA splicing

In eukaryotes, the spliceosome performs the splicing reactions essential for removing intron sequences, this process is required for the formation of mature mRNA. The spliceosome is another RNP often known as the snRNP or tri-snRNP. There are two different forms of the spliceosome, the major and minor forms. The ncRNA components of the major spliceosome are U1, U2, U4, U5, and U6. The ncRNA components of the minor spliceosome are U11, U12, U5, U4atac and U6atac.[citation needed]
Another group of introns can catalyse their own removal from host transcripts; these are called self-splicing RNAs. There are two main groups of self-splicing RNAs: group I catalytic intron and group II catalytic intron. These ncRNAs catalyze their own excision from mRNA, tRNA and rRNA precursors in a wide range of organisms.[citation needed]
In mammals it has been found that snoRNAs can also regulate the alternative splicing of mRNA, for example snoRNA HBII-52 regulates the splicing of serotonin receptor 2C.[32]
In nematodes, the SmY ncRNA appears to be involved in mRNA trans-splicing.[citation needed]
In DNA replication
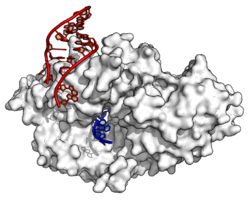
Y RNAs are stem loops, necessary for DNA replication through interactions with chromatin and initiation proteins (including the origin recognition complex).[34][35] They are also components of the Ro60 ribonucleoprotein particle[36] which is a target of autoimmune antibodies in patients with systemic lupus erythematosus.[37]
In gene regulation
The expression of many thousands of genes are regulated by ncRNAs. This regulation can occur in trans or in cis. There is increasing evidence that a special type of ncRNAs called enhancer RNAs, transcribed from the enhancer region of a gene, act to promote gene expression.[citation needed]
Trans-acting
In higher eukaryotes microRNAs regulate gene expression. A single miRNA can reduce the expression levels of hundreds of genes. The mechanism by which mature miRNA molecules act is through partial complementarity to one or more messenger RNA (mRNA) molecules, generally in 3' UTRs. The main function of miRNAs is to down-regulate gene expression.
The ncRNA RNase P has also been shown to influence gene expression. In the human nucleus, RNase P is required for the normal and efficient transcription of various ncRNAs transcribed by RNA polymerase III. These include tRNA, 5S rRNA, SRP RNA, and U6 snRNA genes. RNase P exerts its role in transcription through association with Pol III and chromatin of active tRNA and 5S rRNA genes.[38]
It has been shown that 7SK RNA, a metazoan ncRNA, acts as a negative regulator of the RNA polymerase II elongation factor P-TEFb, and that this activity is influenced by stress response pathways.[citation needed]
The bacterial ncRNA, 6S RNA, specifically associates with RNA polymerase holoenzyme containing the sigma70 specificity factor. This interaction represses expression from a sigma70-dependent promoter during stationary phase.[citation needed]
Another bacterial ncRNA, OxyS RNA represses translation by binding to Shine-Dalgarno sequences thereby occluding ribosome binding. OxyS RNA is induced in response to oxidative stress in Escherichia coli.[citation needed]
The B2 RNA is a small noncoding RNA polymerase III transcript that represses mRNA transcription in response to heat shock in mouse cells. B2 RNA inhibits transcription by binding to core Pol II. Through this interaction, B2 RNA assembles into preinitiation complexes at the promoter and blocks RNA synthesis.[39]
A recent study has shown that just the act of transcription of ncRNA sequence can have an influence on gene expression. RNA polymerase II transcription of ncRNAs is required for chromatin remodelling in the Schizosaccharomyces pombe. Chromatin is progressively converted to an open configuration, as several species of ncRNAs are transcribed.[40]
Cis-acting
A number of ncRNAs are embedded in the 5' UTRs (Untranslated Regions) of protein coding genes and influence their expression in various ways. For example, a riboswitch can directly bind a small target molecule; the binding of the target affects the gene's activity.[citation needed]
RNA leader sequences are found upstream of the first gene of amino acid biosynthetic operons. These RNA elements form one of two possible structures in regions encoding very short peptide sequences that are rich in the end product amino acid of the operon. A terminator structure forms when there is an excess of the regulatory amino acid and ribosome movement over the leader transcript is not impeded. When there is a deficiency of the charged tRNA of the regulatory amino acid the ribosome translating the leader peptide stalls and the antiterminator structure forms. This allows RNA polymerase to transcribe the operon. Known RNA leaders are Histidine operon leader, Leucine operon leader, Threonine operon leader and the Tryptophan operon leader.[citation needed]
Iron response elements (IRE) are bound by iron response proteins (IRP). The IRE is found in UTRs of various mRNAs whose products are involved in iron metabolism. When iron concentration is low, IRPs bind the ferritin mRNA IRE leading to translation repression.[citation needed]
Internal ribosome entry sites (IRES) are RNA structures that allow for translation initiation in the middle of a mRNA sequence as part of the process of protein synthesis.[citation needed]
In genome defense
Piwi-interacting RNAs (piRNAs) expressed in mammalian testes and somatic cells form RNA-protein complexes with Piwi proteins. These piRNA complexes (piRCs) have been linked to transcriptional gene silencing of retrotransposons and other genetic elements in germline cells, particularly those in spermatogenesis.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) are repeats found in the DNA of many bacteria and archaea. The repeats are separated by spacers of similar length. It has been demonstrated that these spacers can be derived from phage and subsequently help protect the cell from infection.
Chromosome structure
Telomerase is an RNP enzyme that adds specific DNA sequence repeats ("TTAGGG" in vertebrates) to telomeric regions, which are found at the ends of eukaryotic chromosomes. The telomeres contain condensed DNA material, giving stability to the chromosomes. The enzyme is a reverse transcriptase that carries Telomerase RNA, which is used as a template when it elongates telomeres, which are shortened after each replication cycle.
Xist (X-inactive-specific transcript) is a long ncRNA gene on the X chromosome of the placental mammals that acts as major effector of the X chromosome inactivation process forming Barr bodies. An antisense RNA, Tsix, is a negative regulator of Xist. X chromosomes lacking Tsix expression (and thus having high levels of Xist transcription) are inactivated more frequently than normal chromosomes. In drosophilids, which also use an XY sex-determination system, the roX (RNA on the X) RNAs are involved in dosage compensation.[41] Both Xist and roX operate by epigenetic regulation of transcription through the recruitment of histone-modifying enzymes.
Bifunctional RNA
Bifunctional RNAs, or dual-function RNAs, are RNAs that have two distinct functions.[42][43] The majority of the known bifunctional RNAs are mRNAs that encode both a protein and ncRNAs. However, a growing number of ncRNAs fall into two different ncRNA categories; e.g., H/ACA box snoRNA and miRNA.[44][45]
Two well known examples of bifunctional RNAs are SgrS RNA and RNAIII. However, a handful of other bifunctional RNAs are known to exist (e.g., steroid receptor activator/SRA,[46] VegT RNA,[47][48] Oskar RNA,[49] ENOD40,[50] p53 RNA[51] SR1 RNA,[52] and Spot 42 RNA.[53]) Bifunctional RNAs were the subject of a 2011 special issue of Biochimie.[54]
As a hormone
There is an important link between certain non-coding RNAs and the control of hormone-regulated pathways. In Drosophila, hormones such as ecdysone and juvenile hormone can promote the expression of certain miRNAs. Furthermore, this regulation occurs at distinct temporal points within Caenorhabditis elegans development.[55] In mammals, miR-206 is a crucial regulator of estrogen-receptor-alpha.[56]
Non-coding RNAs are crucial in the development of several endocrine organs, as well as in endocrine diseases such as diabetes mellitus.[57] Specifically in the MCF-7 cell line, addition of 17β-estradiol increased global transcription of the noncoding RNAs called lncRNAs near estrogen-activated coding genes.[58]
In pathogenic avoidance
C. elegans was shown to learn and inherit pathogenic avoidance after exposure to a single non-coding RNA of a bacterial pathogen.[59][60]
Roles in disease
As with proteins, mutations or imbalances in the ncRNA repertoire within the body can cause a variety of diseases.
Cancer
Many ncRNAs show abnormal expression patterns in cancerous tissues.[6] These include miRNAs, long mRNA-like ncRNAs,[61][62] GAS5,[63] SNORD50,[64] telomerase RNA and Y RNAs.[65] The miRNAs are involved in the large scale regulation of many protein coding genes,[66][67] the Y RNAs are important for the initiation of DNA replication,[34] telomerase RNA that serves as a primer for telomerase, an RNP that extends telomeric regions at chromosome ends (see telomeres and disease for more information). The direct function of the long mRNA-like ncRNAs is less clear.
Germline mutations in miR-16-1 and miR-15 primary precursors have been shown to be much more frequent in patients with chronic lymphocytic leukemia compared to control populations.[68][69]
It has been suggested that a rare SNP (rs11614913) that overlaps hsa-mir-196a-2 has been found to be associated with non-small cell lung carcinoma.[70] Likewise, a screen of 17 miRNAs that have been predicted to regulate a number of breast cancer associated genes found variations in the microRNAs miR-17 and miR-30c-1of patients; these patients were noncarriers of BRCA1 or BRCA2 mutations, lending the possibility that familial breast cancer may be caused by variation in these miRNAs.[71] The p53 tumor suppressor is arguably the most important agent in preventing tumor formation and progression. The p53 protein functions as a transcription factor with a crucial role in orchestrating the cellular stress response. In addition to its crucial role in cancer, p53 has been implicated in other diseases including diabetes, cell death after ischemia, and various neurodegenerative diseases such as Huntington, Parkinson, and Alzheimer. Studies have suggested that p53 expression is subject to regulation by non-coding RNA.[5]
Another example of non-coding RNA dysregulated in cancer cells is the long non-coding RNA Linc00707. Linc00707 is upregulated and sponges miRNAs in human bone marrow-derived mesenchymal stem cells,[72] in hepatocellular carcinoma,[73] gastric cancer[74] or breast cancer,[75][76] and thus promotes osteogenesis, contributes to hepatocellular carcinoma progression, promotes proliferation and metastasis, or indirectly regulates expression of proteins involved in cancer aggressiveness, respectively.
Prader–Willi syndrome
The deletion of the 48 copies of the C/D box snoRNA SNORD116 has been shown to be the primary cause of Prader–Willi syndrome.[77][78][79][80] Prader–Willi is a developmental disorder associated with over-eating and learning difficulties. SNORD116 has potential target sites within a number of protein-coding genes, and could have a role in regulating alternative splicing.[81]
Autism
The chromosomal locus containing the small nucleolar RNA SNORD115 gene cluster has been duplicated in approximately 5% of individuals with autistic traits.[82][83] A mouse model engineered to have a duplication of the SNORD115 cluster displays autistic-like behaviour.[84] A recent small study of post-mortem brain tissue demonstrated altered expression of long non-coding RNAs in the prefrontal cortex and cerebellum of autistic brains as compared to controls.[85]
Cartilage–hair hypoplasia
Mutations within RNase MRP have been shown to cause cartilage–hair hypoplasia, a disease associated with an array of symptoms such as short stature, sparse hair, skeletal abnormalities and a suppressed immune system that is frequent among Amish and Finland .[86][87][88] The best characterised variant is an A-to-G transition at nucleotide 70 that is in a loop region two bases 5' of a conserved pseudoknot. However, many other mutations within RNase MRP also cause CHH.
Alzheimer's disease
The antisense RNA, BACE1-AS is transcribed from the opposite strand to BACE1 and is upregulated in patients with Alzheimer's disease.[89] BACE1-AS regulates the expression of BACE1 by increasing BACE1 mRNA stability and generating additional BACE1 through a post-transcriptional feed-forward mechanism. By the same mechanism it also raises concentrations of beta amyloid, the main constituent of senile plaques. BACE1-AS concentrations are elevated in subjects with Alzheimer's disease and in amyloid precursor protein transgenic mice.
miR-96 and hearing loss
Variation within the seed region of mature miR-96 has been associated with autosomal dominant, progressive hearing loss in humans and mice. The homozygous mutant mice were profoundly deaf, showing no cochlear responses. Heterozygous mice and humans progressively lose the ability to hear.[90][91][92]
Mitochondrial transfer RNAs
A number of mutations within mitochondrial tRNAs have been linked to diseases such as MELAS syndrome, MERRF syndrome, and chronic progressive external ophthalmoplegia.[93][94][95][96]
Distinction between functional RNA (fRNA) and ncRNA
Scientists have started to distinguish functional RNA (fRNA) from ncRNA, to describe regions functional at the RNA level that may or may not be stand-alone RNA transcripts.[97][98][99] This implies that fRNA (such as riboswitches, SECIS elements, and other cis-regulatory regions) is not ncRNA. Yet fRNA could also include mRNA, as this is RNA coding for protein, and hence is functional. Additionally artificially evolved RNAs also fall under the fRNA umbrella term. Some publications[24] state that ncRNA and fRNA are nearly synonymous, however others have pointed out that a large proportion of annotated ncRNAs likely have no function.[9][10] It also has been suggested to simply use the term RNA, since the distinction from a protein coding RNA (messenger RNA) is already given by the qualifier mRNA.[100] This eliminates the ambiguity when addressing a gene "encoding a non-coding" RNA. Besides, there may be a number of ncRNAs that are misannoted in published literature and datasets.[101][102][103]
See also
- Extracellular RNA
- List of RNAs
- Nucleic acid structure
- Rfam
- Riboswitch
- Ribozyme
- RNAs present in environmental samples
- VA (viral associated) RNA
References
- ↑ "Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution". Science 308 (5725): 1149–1154. May 2005. doi:10.1126/science.1108625. PMID 15790807. Bibcode: 2005Sci...308.1149C.
- ↑ "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Nature 447 (7146): 799–816. June 2007. doi:10.1038/nature05874. PMID 17571346. Bibcode: 2007Natur.447..799B.
- ↑ "Demystifying emerging bulk RNA-Seq applications: the application and utility of bioinformatic methodology". Briefings in Bioinformatics 22 (6). November 2021. doi:10.1093/bib/bbab259. PMID 34329375.
- ↑ "Structured RNAs in the ENCODE selected regions of the human genome". Genome Research 17 (6): 852–864. June 2007. doi:10.1101/gr.5650707. PMID 17568003.
- ↑ 5.0 5.1 Non-coding RNAs and Epigenetic Regulation of Gene Expression: Drivers of Natural Selection. Caister Academic Press. 2012. ISBN 978-1-904455-94-3.
- ↑ 6.0 6.1 "The non-coding oncogene: a case of missing DNA evidence?". Frontiers in Genetics 3: 170. 2012. doi:10.3389/fgene.2012.00170. PMID 22988449.
- ↑ "Most "dark matter" transcripts are associated with known genes". PLOS Biology 8 (5): e1000371. May 2010. doi:10.1371/journal.pbio.1000371. PMID 20502517.
- ↑ "Non-coding RNAs: hope or hype?". Trends in Genetics 21 (5): 289–297. May 2005. doi:10.1016/j.tig.2005.03.007. PMID 15851066.
- ↑ 9.0 9.1 "Waste not, want not--transcript excess in multicellular eukaryotes". Trends in Genetics 21 (5): 287–288. May 2005. doi:10.1016/j.tig.2005.02.014. PMID 15851065.
- ↑ 10.0 10.1 "Non-coding RNA: what is functional and what is junk?". Frontiers in Genetics 6: 2. 2015. doi:10.3389/fgene.2015.00002. PMID 25674102.
- ↑ RNA, The Epicenter of Genetic Information : A New Understanding of Molecular Biology. CRC Press. 2022. ISBN 9780367623920.
- ↑ "Long Noncoding RNAs and Repetitive Elements: Junk or Intimate Evolutionary Partners?". Trends in Genetics 35 (12): 892–902. December 2019. doi:10.1016/j.tig.2019.09.006. PMID 31662190.
- ↑ "Friedrich Miescher and the discovery of DNA". Developmental Biology 278 (2): 274–288. February 2005. doi:10.1016/j.ydbio.2004.11.028. PMID 15680349.
- ↑ "Pentose nucleotides in the cytoplasm of growing tissues". Nature 143 (3623): 602–3. 1939. doi:10.1038/143602c0. Bibcode: 1939Natur.143..602C.
- ↑ "On protein synthesis". Symposia of the Society for Experimental Biology 12: 138–163. 1958. PMID 13580867.
- ↑ 16.0 16.1 "Structure of a Ribonucleic Acid". Science 147 (3664): 1462–1465. March 1965. doi:10.1126/science.147.3664.1462. PMID 14263761. Bibcode: 1965Sci...147.1462H.
- ↑ 17.0 17.1 "The Nobel Prize in Physiology or Medicine 1968". Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1968/index.html.
- ↑ "Nucleotide sequence of a yeast tyrosine transfer RNA". Science 153 (3735): 531–534. July 1966. doi:10.1126/science.153.3735.531. PMID 5938777. Bibcode: 1966Sci...153..531M.
- ↑ "Serine specific transfer ribonucleic acids. XIV. Comparison of nucleotide sequences and secondary structure models". Cold Spring Harbor Symposia on Quantitative Biology 31: 417–424. 1966. doi:10.1101/SQB.1966.031.01.054. PMID 5237198.
- ↑ "Primary structure of wheat germ phenylalanine transfer RNA". Proceedings of the National Academy of Sciences of the United States of America 62 (3): 941–945. March 1969. doi:10.1073/pnas.62.3.941. PMID 5257014. Bibcode: 1969PNAS...62..941D.
- ↑ "On the conformation of transfer RNA". Proceedings of the National Academy of Sciences of the United States of America 61 (4): 1384–1391. December 1968. doi:10.1073/pnas.61.4.1384. PMID 4884685. Bibcode: 1968PNAS...61.1384C.
- ↑ "Structure of yeast phenylalanine transfer RNA at 2.5 A resolution". Proceedings of the National Academy of Sciences of the United States of America 72 (11): 4414–4418. November 1975. doi:10.1073/pnas.72.11.4414. PMID 1105583. Bibcode: 1975PNAS...72.4414L.
- ↑ "Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain". Science 179 (4070): 285–288. January 1973. doi:10.1126/science.179.4070.285. PMID 4566654. Bibcode: 1973Sci...179..285K.
- ↑ 24.0 24.1 "Non-coding RNA genes and the modern RNA world". Nature Reviews. Genetics 2 (12): 919–929. December 2001. doi:10.1038/35103511. PMID 11733745.
- ↑ "Advanced Information: RNA interference". The Nobel Prize in Physiology or Medicine 2006. http://nobelprize.org/nobel_prizes/medicine/laureates/2006/adv.html.
- ↑ "Computational Identification of piRNAs Using Features Based on RNA Sequence, Structure, Thermodynamic and Physicochemical Properties". Current Genomics 20 (7): 508–518. November 2019. doi:10.2174/1389202920666191129112705. PMID 32655289.
- ↑ "Relics from the RNA world". Journal of Molecular Evolution 46 (1): 18–36. January 1998. doi:10.1007/PL00006280. PMID 9419222. Bibcode: 1998JMolE..46...18J.
- ↑ "The path from the RNA world". Journal of Molecular Evolution 46 (1): 1–17. January 1998. doi:10.1007/PL00006275. PMID 9419221. Bibcode: 1998JMolE..46....1P.
- ↑ "Early evolution: prokaryotes, the new kids on the block". BioEssays 21 (10): 880–889. October 1999. doi:10.1002/(SICI)1521-1878(199910)21:10<880::AID-BIES11>3.0.CO;2-P. PMID 10497339.
- ↑ "The complete atomic structure of the large ribosomal subunit at 2.4 A resolution". Science 289 (5481): 905–920. August 2000. doi:10.1126/science.289.5481.905. PMID 10937989. Bibcode: 2000Sci...289..905B.
- ↑ "Sequence analysis of RNase MRP RNA reveals its origination from eukaryotic RNase P RNA". RNA 12 (5): 699–706. May 2006. doi:10.1261/rna.2284906. PMID 16540690.
- ↑ "The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C". Science 311 (5758): 230–232. January 2006. doi:10.1126/science.1118265. PMID 16357227. Bibcode: 2006Sci...311..230K.
- ↑ "Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity". Cell 121 (4): 529–539. May 2005. doi:10.1016/j.cell.2005.03.009. PMID 15907467.
- ↑ 34.0 34.1 "Functional requirement of noncoding Y RNAs for human chromosomal DNA replication". Molecular and Cellular Biology 26 (18): 6993–7004. September 2006. doi:10.1128/MCB.01060-06. PMID 16943439.
- ↑ "Dynamic interaction of Y RNAs with chromatin and initiation proteins during human DNA replication". Journal of Cell Science 124 (Pt 12): 2058–2069. June 2011. doi:10.1242/jcs.086561. PMID 21610089.
- ↑ "Y RNAs: recent developments". Biomolecular Concepts 4 (2): 103–110. April 2013. doi:10.1515/bmc-2012-0050. PMID 25436569.
- ↑ "Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus". Science 211 (4480): 400–402. January 1981. doi:10.1126/science.6164096. PMID 6164096. Bibcode: 1981Sci...211..400L.
- ↑ "A role for the catalytic ribonucleoprotein RNase P in RNA polymerase III transcription". Genes & Development 20 (12): 1621–1635. June 2006. doi:10.1101/gad.386706. PMID 16778078.
- ↑ "B2 RNA binds directly to RNA polymerase II to repress transcript synthesis". Nature Structural & Molecular Biology 11 (9): 822–829. September 2004. doi:10.1038/nsmb812. PMID 15300239.
- ↑ "Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs". Nature 456 (7218): 130–134. November 2008. doi:10.1038/nature07348. PMID 18820678. Bibcode: 2008Natur.456..130H.
- ↑ "Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins". Science 298 (5598): 1620–1623. November 2002. doi:10.1126/science.1076686. PMID 12446910. Bibcode: 2002Sci...298.1620P.
- ↑ "A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide". Proceedings of the National Academy of Sciences of the United States of America 104 (51): 20454–20459. December 2007. doi:10.1073/pnas.0708102104. PMID 18042713. Bibcode: 2007PNAS..10420454W.
- ↑ "Differentiating protein-coding and noncoding RNA: challenges and ambiguities". PLOS Computational Biology 4 (11): e1000176. November 2008. doi:10.1371/journal.pcbi.1000176. PMID 19043537. Bibcode: 2008PLSCB...4E0176D.
- ↑ "snoRNA, a novel precursor of microRNA in Giardia lamblia". PLOS Pathogens 4 (11): e1000224. November 2008. doi:10.1371/journal.ppat.1000224. PMID 19043559.
- ↑ "A human snoRNA with microRNA-like functions". Molecular Cell 32 (4): 519–528. November 2008. doi:10.1016/j.molcel.2008.10.017. PMID 19026782.
- ↑ "Steroid receptor RNA activator (SRA1): unusual bifaceted gene products with suspected relevance to breast cancer". Nuclear Receptor Signaling 5: e006. August 2007. doi:10.1621/nrs.05006. PMID 17710122.
- ↑ "Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning". Development 122 (12): 4119–4129. December 1996. doi:10.1242/dev.122.12.4119. PMID 9012531.
- ↑ "Potential structural role of non-coding and coding RNAs in the organization of the cytoskeleton at the vegetal cortex of Xenopus oocytes". Development 132 (15): 3445–3457. August 2005. doi:10.1242/dev.01919. PMID 16000384.
- ↑ "A translation-independent role of oskar RNA in early Drosophila oogenesis". Development 133 (15): 2827–2833. August 2006. doi:10.1242/dev.02456. PMID 16835436.
- ↑ "Identification of conserved secondary structures and expansion segments in enod40 RNAs reveals new enod40 homologues in plants". Nucleic Acids Research 35 (9): 3144–3152. 2007. doi:10.1093/nar/gkm173. PMID 17452360.
- ↑ "P53 mRNA controls p53 activity by managing Mdm2 functions". Nature Cell Biology 10 (9): 1098–1105. September 2008. doi:10.1038/ncb1770. PMID 19160491.
- ↑ "SR1--a small RNA with two remarkably conserved functions". Nucleic Acids Research 40 (22): 11659–11672. December 2012. doi:10.1093/nar/gks895. PMID 23034808.
- ↑ "Dual-function Spot 42 RNA encodes a 15-amino acid protein that regulates the CRP transcription factor". Proceedings of the National Academy of Sciences of the United States of America 119 (10): e2119866119. March 2022. doi:10.1073/pnas.2119866119. PMID 35239441. Bibcode: 2022PNAS..11919866A.
- ↑ "Coding or non-coding: Need they be exclusive?". Biochimie 93 (11): vi-vii. November 2011. doi:10.1016/S0300-9084(11)00322-1. PMID 21963143. https://zenodo.org/record/889579.
- ↑ "Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity". Developmental Biology 259 (1): 9–18. July 2003. doi:10.1016/S0012-1606(03)00208-2. PMID 12812784.
- ↑ "The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines". Molecular Endocrinology 21 (5): 1132–1147. May 2007. doi:10.1210/me.2007-0022. PMID 17312270.
- ↑ "Long non-coding RNAs as regulators of the endocrine system". Nature Reviews. Endocrinology 11 (3): 151–160. March 2015. doi:10.1038/nrendo.2014.229. PMID 25560704.
- ↑ "Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation". Nature 498 (7455): 516–520. June 2013. doi:10.1038/nature12210. PMID 23728302. Bibcode: 2013Natur.498..516L.
- ↑ "Researchers discover how worms pass knowledge of a pathogen to offspring" (in en). phys.org. https://phys.org/news/2020-09-worms-knowledge-pathogen-offspring.html.
- ↑ "C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance". Nature 586 (7829): 445–451. October 2020. doi:10.1038/s41586-020-2699-5. PMID 32908307. Bibcode: 2020Natur.586..445K.
- ↑ "Cloning of the mRNA of overexpression in colon carcinoma-1: a sequence overexpressed in a subset of colon carcinomas". Cancer Genetics and Cytogenetics 133 (1): 55–60. February 2002. doi:10.1016/S0165-4608(01)00634-3. PMID 11890990.
- ↑ "Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1". DNA and Cell Biology 25 (3): 135–141. March 2006. doi:10.1089/dna.2006.25.135. PMID 16569192.
- ↑ "GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer". Oncogene 28 (2): 195–208. January 2009. doi:10.1038/onc.2008.373. PMID 18836484.
- ↑ "Implication of snoRNA U50 in human breast cancer". Journal of Genetics and Genomics = Yi Chuan Xue Bao 36 (8): 447–454. August 2009. doi:10.1016/S1673-8527(08)60134-4. PMID 19683667.
- ↑ "Noncoding human Y RNAs are overexpressed in tumours and required for cell proliferation". British Journal of Cancer 98 (5): 981–988. March 2008. doi:10.1038/sj.bjc.6604254. PMID 18283318.
- ↑ "The widespread impact of mammalian MicroRNAs on mRNA repression and evolution". Science 310 (5755): 1817–1821. December 2005. doi:10.1126/science.1121158. PMID 16308420. Bibcode: 2005Sci...310.1817F.
- ↑ "Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs". Nature 433 (7027): 769–773. February 2005. doi:10.1038/nature03315. PMID 15685193. Bibcode: 2005Natur.433..769L.
- ↑ "A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia". The New England Journal of Medicine 353 (17): 1793–1801. October 2005. doi:10.1056/NEJMoa050995. PMID 16251535.
- ↑ "Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia". Proceedings of the National Academy of Sciences of the United States of America 99 (24): 15524–15529. November 2002. doi:10.1073/pnas.242606799. PMID 12434020. Bibcode: 2002PNAS...9915524C.
- ↑ "Genetic variants of miRNA sequences and non-small cell lung cancer survival". The Journal of Clinical Investigation 118 (7): 2600–2608. July 2008. doi:10.1172/JCI34934. PMID 18521189.
- ↑ "Novel genetic variants in microRNA genes and familial breast cancer". International Journal of Cancer 124 (5): 1178–1182. March 2009. doi:10.1002/ijc.24008. PMID 19048628.
- ↑ "Long noncoding RNA LINC00707 sponges miR-370-3p to promote osteogenesis of human bone marrow-derived mesenchymal stem cells through upregulating WNT2B". Stem Cell Research & Therapy 10 (1): 67. February 2019. doi:10.1186/s13287-019-1161-9. PMID 30795799.
- ↑ "LINC00707 contributes to hepatocellular carcinoma progression via sponging miR-206 to increase CDK14". Journal of Cellular Physiology 234 (7): 10615–10624. July 2019. doi:10.1002/jcp.27737. PMID 30488589.
- ↑ "The long intergenic non-protein coding RNA 707 promotes proliferation and metastasis of gastric cancer by interacting with mRNA stabilizing protein HuR". Cancer Letters 443: 67–79. February 2019. doi:10.1016/j.canlet.2018.11.032. PMID 30502359.
- ↑ "MicroRNA-876 is sponged by long noncoding RNA LINC00707 and directly targets metadherin to inhibit breast cancer malignancy". Cancer Management and Research 11: 5255–5269. 2019-06-06. doi:10.2147/cmar.s210845. PMID 31239777.
- ↑ "Linc00707 promotes cell proliferation, invasion, and migration via the miR-30c/CTHRC1 regulatory loop in breast cancer". European Review for Medical and Pharmacological Sciences 24 (9): 4863–4872. May 2020. doi:10.26355/eurrev_202005_21175. PMID 32432749.
- ↑ "Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster". Nature Genetics 40 (6): 719–721. June 2008. doi:10.1038/ng.158. PMID 18500341.
- ↑ "Deletion of the MBII-85 snoRNA gene cluster in mice results in postnatal growth retardation". PLOS Genetics 3 (12): e235. December 2007. doi:10.1371/journal.pgen.0030235. PMID 18166085.
- ↑ "SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice". PLOS ONE 3 (3): e1709. March 2008. doi:10.1371/journal.pone.0001709. PMID 18320030. Bibcode: 2008PLoSO...3.1709D.
- ↑ "Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader-Willi syndrome mouse models". Mammalian Genome 16 (6): 424–431. June 2005. doi:10.1007/s00335-005-2460-2. PMID 16075369.
- ↑ "snoTARGET shows that human orphan snoRNA targets locate close to alternative splice junctions". Gene 408 (1–2): 172–179. January 2008. doi:10.1016/j.gene.2007.10.037. PMID 18160232.
- ↑ "Chromosome 15q11-13 abnormalities and other medical conditions in individuals with autism spectrum disorders". Psychiatric Genetics 14 (3): 131–137. September 2004. doi:10.1097/00041444-200409000-00002. PMID 15318025.
- ↑ "Copy-number variations associated with neuropsychiatric conditions". Nature 455 (7215): 919–923. October 2008. doi:10.1038/nature07458. PMID 18923514. Bibcode: 2008Natur.455..919C.
- ↑ "Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism". Cell 137 (7): 1235–1246. June 2009. doi:10.1016/j.cell.2009.04.024. PMID 19563756.
- ↑ "Aberrant expression of long noncoding RNAs in autistic brain". Journal of Molecular Neuroscience 49 (3): 589–593. March 2013. doi:10.1007/s12031-012-9880-8. PMID 22949041.
- ↑ "Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia". Cell 104 (2): 195–203. January 2001. doi:10.1016/S0092-8674(01)00205-7. PMID 11207361.
- ↑ "RNase MRP RNA and human genetic diseases". Cell Research 17 (3): 219–226. March 2007. doi:10.1038/sj.cr.7310120. PMID 17189938.
- ↑ "Variability of clinical and laboratory features among patients with ribonuclease mitochondrial RNA processing endoribonuclease gene mutations". The Journal of Allergy and Clinical Immunology 122 (6): 1178–1184. December 2008. doi:10.1016/j.jaci.2008.07.036. PMID 18804272.
- ↑ "Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase". Nature Medicine 14 (7): 723–730. July 2008. doi:10.1038/nm1784. PMID 18587408.
- ↑ "Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss". Nature Genetics 41 (5): 609–613. May 2009. doi:10.1038/ng.355. PMID 19363479.
- ↑ "An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice". Nature Genetics 41 (5): 614–618. May 2009. doi:10.1038/ng.369. PMID 19363478.
- ↑ "Little but loud: small RNAs have a resounding affect on ear development". Brain Research 1277: 104–114. June 2009. doi:10.1016/j.brainres.2009.02.027. PMID 19245798.
- ↑ "Mitochondrial DNA mutations in human disease". Nature Reviews. Genetics 6 (5): 389–402. May 2005. doi:10.1038/nrg1606. PMID 15861210.
- ↑ "Mitochondrial tRNA mutations and disease". Wiley Interdisciplinary Reviews. RNA 1 (2): 304–324. September 2010. doi:10.1002/wrna.27. PMID 21935892.
- ↑ "Mitochondrial tRNA mutations: clinical and functional perturbations". RNA Biology 4 (1): 38–66. January 2007. doi:10.4161/rna.4.1.4548. PMID 17617745.
- ↑ "Transfer RNA and human disease". Frontiers in Genetics 5: 158. 2014. doi:10.3389/fgene.2014.00158. PMID 24917879.
- ↑ "A computational approach to identify genes for functional RNAs in genomic sequences". Nucleic Acids Research 29 (19): 3928–3938. October 2001. doi:10.1093/nar/29.19.3928. PMID 11574674.
- ↑ "Identification and classification of conserved RNA secondary structures in the human genome". PLOS Computational Biology 2 (4): e33. April 2006. doi:10.1371/journal.pcbi.0020033. PMID 16628248. Bibcode: 2006PLSCB...2...33P.
- ↑ "GraphDNA: a Java program for graphical display of DNA composition analyses". BMC Bioinformatics 8: 21. January 2007. doi:10.1186/1471-2105-8-21. PMID 17244370.
- ↑ "What is an RNA? A top layer for RNA classification". RNA Biology 13 (2): 140–144. February 2015. doi:10.1080/15476286.2015.1128064. PMID 26818079.
- ↑ "Many lncRNAs, 5'UTRs, and pseudogenes are translated and some are likely to express functional proteins". eLife 4: e08890. December 2015. doi:10.7554/eLife.08890. PMID 26687005.
- ↑ "Non-coding RNA fragments account for the majority of annotated piRNAs expressed in somatic non-gonadal tissues" (in En). Communications Biology 1 (1): 2. 2018-01-22. doi:10.1038/s42003-017-0001-7. PMID 30271890.
- ↑ "Methods for distinguishing between protein-coding and long noncoding RNAs and the elusive biological purpose of translation of long noncoding RNAs". Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1859 (1): 31–40. January 2016. doi:10.1016/j.bbagrm.2015.07.017. PMID 26265145.
External links
- "RNAdb". http://jsm-research.imb.uq.edu.au/rnadb/. "This database is a comprehensive mammalian noncoding RNA database (RNAdb)"
- The Rfam Database — a curated list of hundreds of families of related ncRNAs
- NONCODE.org — a free database of all kinds of noncoding RNAs (except tRNAs and rRNAs)
- RNAcon Prediction and classification of ncRNA BMC Genomics 2014, 15:127
- ENCODE threads explorer Non-coding RNA characterization. Nature (journal)
- The Non-coding RNA Databases Resource (NRDR) — a curated source of data related to over non-coding RNA databases available over the internet
- DASHR - a database of small non-coding RNAs Bioinformatics 2018
 |
