Biology:Calcium imaging
Calcium imaging is a microscopy technique to optically measure the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging takes advantage of calcium indicators, fluorescent molecules that respond to the binding of Ca2+ ions by fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI).[1] This technique has allowed studies of calcium signalling in a wide variety of cell types. In neurons, action potential generation is always accompanied by rapid influx of Ca2+ ions. Thus, calcium imaging can be used to monitor the electrical activity in hundreds of neurons in cell culture or in living animals, which has made it possible to observe the activity of neuronal circuits during ongoing behavior.[2]
Chemical indicators
Chemical indicators are small molecules that can chelate calcium ions. All these molecules are based on an EGTA homologue called BAPTA, with high selectivity for calcium (Ca2+) ions versus magnesium (Mg2+) ions.
This group of indicators includes fura-2, indo-1, fluo-3, fluo-4, Calcium Green-1.
These dyes are often used with the chelator carboxyl groups masked as acetoxymethyl esters, in order to render the molecule lipophilic and to allow easy entrance into the cell. Once this form of the indicator is in the cell, cellular esterases will free the carboxyl groups and the indicator will be able to bind calcium. The free acid form of the dyes (i.e. without the acetoxymethyl ester modification) can also be directly injected into cells via a microelectrode or micropipette which removes uncertainties as to the cellular compartment holding the dye (the acetoxymethyl ester can also enter the endoplasmic reticulum and mitochondria). Binding of a Ca2+ ion to a fluorescent indicator molecule leads to either an increase in quantum yield of fluorescence or emission/excitation wavelength shift. Individual chemical Ca2+ fluorescent indicators are utilized for cytosolic calcium measurements in a wide variety of cellular preparations. The first real time (video rate) Ca2+ imaging was carried out in 1986 in cardiac cells using intensified video cameras.[3] Later development of the technique using laser scanning confocal microscopes revealed sub-cellular Ca2+ signals in the form of Ca2+ sparks and Ca2+ blips. Relative responses from a combination of chemical Ca2+ fluorescent indicators were also used to quantify calcium transients in intracellular organelles such as mitochondria.[4]
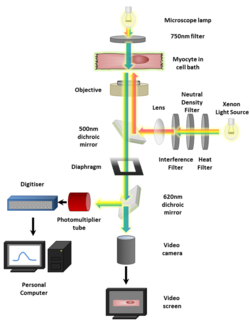
Calcium imaging, also referred to as calcium mapping, is also used to perform research on myocardial tissue.[5] Calcium mapping is a ubiquitous technique used on whole, isolated hearts such as mouse, rat, and rabbit species.
Genetically encoded calcium indicators
Genetically encoded calcium indicators (GECIs) are powerful tools useful for in vivo imaging of cellular, developmental, and physiological processes.[6][7][8][9] GECIs do not need to be acutely loaded into cells; instead the genes encoding for these proteins can be introduced into individual cells or cell lines by various transfection methods. It is also possible to create transgenic animals expressing the indicator in all cells or selectively in certain cellular subtypes. GECIs are used to study neurons,[10][11] T-cells,[12] cardiomyocytes,[13] and other cell types. Some GECIs report calcium by direct emission of photons (luminescence), but most rely on fluorescent proteins as reporters, including the green fluorescent protein GFP and its variants (eGFP, YFP, CFP).
Of the fluorescent reporters, calcium indicator systems can be classified into single fluorescent protein (FP) systems, and paired fluorescent protein systems. Camgaroos were one of the first developed variants involving a single protein system. Camgaroos take advantage of calmodulin (CaM), a calcium binding protein. In these structures, CaM is inserted in the middle of yellow fluorescent protein (YFP) at Y145. Previous mutagenesis studies revealed that mutations at this position conferred pH stability while maintaining fluorescent properties, making Y145 an insertion point of interest. Additionally, the N and C termini of YFP are linked by a peptide linker (GGTGGS). When CaM binds to Ca2+, the effective pKa is lowered, allowing for chromophore deprotonation.[14] This results in increased fluorescence upon calcium binding in an intensiometric fashion. Such detection is in contrast with ratiometric systems, in which there is a change in the absorbance/emission spectra as a result of Ca2+ binding.[15] A later developed single-FP system, dubbed G-CaMP, also invokes circularly permuted GFP. One of the termini is fused with CaM, and the other termini is fused with M13 (the calmodulin binding domain of myosin light kinase)[16] The protein is designed such that the termini are close in space, allowing for Ca2+ binding to cause conformational changes and chromophore modulation, allowing for increased fluorescence. G-CaMP and its refined variants have nanomolar binding affinities.[17] A final single protein variant is the CatchER, which is generally considered to be a lower affinity indicator. Its calcium binding pocket is quite negative; binding of the cation helps to shield the large concentration of negative charge and allows for recovered fluorescence.[18]
In contrast to these systems are paired fluorescent protein systems, which include the prototypical Cameleons. Cameleons consist of two different fluorescent proteins, CaM, M13, and a glycylglycine linker.[15] In the absence of Ca2+, only the donor blue-shifted fluorescent protein will be fluorescent. However, a conformational change caused by calcium binding repositions the red-shifted fluorescent protein, allowing for FRET (Förster resonance energy transfer) to take place. Cameleon indicators produce a ratiometric signal (i.e. the measured FRET efficiency depends on the calcium concentration). Original variants of cameleons were originally more sensitive to Ca2+ and were acid quenched.[19] Such shortcomings were abrogated by Q69K and V68L mutations. Both of these residues were close to the buried anionic chromophore and these mutations probably hinder protonation, conferring greater pH resistance.
Of growing importance in calcium detection are near-IR (NIR) GECIs, which may open up avenues for multiplexing different indicator systems and allowing deeper tissue penetration. NIRs rely on biliverdin-binding fluorescent proteins, which are largely derived from bacterial phytochromes. NIR systems are similar to inverse pericams in that both experience a decrease in fluorescence upon Ca2+ binding. RCaMPs and RGECOs are functional at 700+ nm, but are quite dim.[20] A Cameleon analog involving NIR FRET has been successfully constructed as well.[21]
A special class of GECIs are designed to form a permanent fluorescent tag in active neurons. They are based on the photoswitchable protein Eos which turns from green to red through photocatalyzed (with violet light) backbone cleavage.[22] Combined with the CaM, violet light photoconverts only neurons that have elevated calcium levels. SynTagMA is a synapse-targeted version of CaMPARI2.[23]
While fluorescent systems are widely used, bioluminescent Ca2+ reporters may also hold potential because of their ability to abrogate autofluorescence, photobleaching [no excitation wavelength is needed], biological degradation and toxicity, in addition to higher signal-to-noise ratios.[24] Such systems may rely on aequorin and the luciferin coelenterazine. Ca2+ binding causes a conformational change that facilitates coelenterazine oxidation. The resultant photoproduct emits blue light as it returns to the ground state. Colocalization of aequorin with GFP facilitates BRET/CRET (Bioluminescence or Chemiluminescence Resonance Energy Transfer),[18] resulting in a 19 - 65 brightness increase. Such structures can be used to probe millimolar to nanomolar calcium concentrations. A similar system invokes obelin and its luciferin coelenteramide, which may possess faster calcium response time and Mg2+ insensitivity than its aqueorin counterpart.[25] Such systems can also leverage the self-assembly of luciferase components. In a system dubbed “nano-lantern,” the luciferase RLuc8 is split and placed on different ends of CaM. Calcium binding brings the RLuc8 components in close proximity, reforming luciferase, and allowing it to transfer to an acceptor fluorescent protein.
To minimize damage to the visualized cells, two-photon microscopy is often invoked to detect the fluorescence from the reporters.[26] The use of near-IR wavelengths and minimization of axial spread of the point function[27] allows for nanometer resolution and deep penetration into the tissue. The dynamic range is often determined from such measurements. For non-ratiometric indicators (typically single protein indicators), it is the ratio of the fluorescence intensities obtained under Ca2+ saturated and depleted conditions, respectively. However, for ratiometric indicators, the dynamic range is the ratio of the maximum FRET efficiency ratio (calcium saturated) to the minimum FRET efficiency ratio (calcium depleted). Yet another common quantity used to measure signals produced by calcium concentration fluxes is the signal-to-baseline ratio (SBR), which is simply the ratio of the change in fluorescence (F - F0) over the baseline fluorescence. This can be related to the SNR (signal to noise ratio) by multiplying the SBR by the square root of the number of counted photons.[18]
| GECI | Year | Sensing | Reporting | Precursor |
|---|---|---|---|---|
| Cameleons[28] | 1997 | Calmodulin | FRET pair: BFP or CFP, and GFP or YFP | - |
| 1997 | Calmodulin | FRET pair: BFP and RFP | - | |
| Pericams[29] | 2000 | Calmodulin | cpGFP | - |
| GCaMP[17][30] | 2000 | Calmodulin | cpEGFP | - |
| TN-L15[31] | 2004 | Modified chicken skeletal muscle troponin C | FRET pair: YFP (Citrine) and CFP (Cerulean) | - |
| TN-humTnC[31] | 2004 | Human cardiac troponin C | FRET pair: YFP (Citrine) and CFP (Cerulean) | - |
| TN-XL[32] | 2006 | Modified chicken skeletal muscle troponin C | FRET pair: permuted YFP (Citrine) and CFP (Cerulean) | TN-L15 |
| TN-XXL[33] | 2008 | Modified csTnC in TN-XL | FRET pair: permuted YFP (Citrine) and CFP (Cerulean) | TN-XL |
| Twitch's[34] | 2014 | Troponin C | FRET pair (various of two FPs) | - |
| RCaMP1[35] | 2013 | Calmodulin | mRuby (red FP) | - |
| jRGECO1a[36] | 2016 | Calmodulin | mApple (red FP) | R-GECO[37] |
A special class of genetically encoded calcium indicators are designed to form a permanent fluorescent tag in active neurons. They are based on the photoswitchable protein mEos which turns from green to red when illuminated with violet light. Combined with the calcium sensor calmodulin, violet light photoconverts only neurons that have elevated calcium levels. SynTagMA is a synapse-targeted version of CaMPARI2.
| GECI | Year | Sensing | Reporting | Precursor |
|---|---|---|---|---|
| CaMPARI[23] | 2015 | Calmodulin + violet light | mEos: green to red conversion | - |
| CaMPARI2[38] | 2018 | Calmodulin + violet light | mEos: green to red conversion | CaMPARI |
| SynTagMA[39] | 2020 | Calmodulin + violet light | mEos: green to red conversion | CaMPARI2 |
| TubuTag[40] | 2021 | Calmodulin + violet light | mEos: green to red conversion | CaMPARI2 |
Usage
Regardless of the type of indicator used the imaging procedure is generally very similar. Cells loaded with an indicator, or expressing it in the case of a GECI, can be viewed using a fluorescence microscope and captured by a Scientific CMOS (sCMOS)[41] camera or CCD camera. Confocal and two-photon microscopes provide optical sectioning ability so that calcium signals can be resolved in microdomains such as dendritic spines or synaptic boutons, even in thick samples such as mammalian brains. Images are analyzed by measuring fluorescence intensity changes for a single wavelength or two wavelengths expressed as a ratio (ratiometric indicators). If necessary, the derived fluorescence intensities and ratios may be plotted against calibrated values for known Ca2+ levels to measure absolute Ca2+ concentrations. Light field microscopy methods[42] extend functional readout of neural activity capabilities in 3D volumes.
Methods such as fiber photometry,[43][44] miniscopes[45] and two-photon microscopy[46][47] offer calcium imaging in freely behaving and head-fixed animal models.
References
- ↑ de Melo Reis, Ricardo Augusto; Freitas, Hércules Rezende; de Mello, Fernando Garcia (2020). "Cell Calcium Imaging as a Reliable Method to Study Neuron–Glial Circuits". Frontiers in Neuroscience 14: 975. doi:10.3389/fnins.2020.569361. ISSN 1662-453X. PMID 33122991.
- ↑ Siciliano, Cody A.; Tye, Kay M. (February 2019). "Leveraging calcium imaging to illuminate circuit dysfunction in addiction". Alcohol (Fayetteville, N.Y.) 74: 47–63. doi:10.1016/j.alcohol.2018.05.013. ISSN 1873-6823. PMID 30470589. PMC 7575247. https://pubmed.ncbi.nlm.nih.gov/30470589/.
- ↑ "Intracellular calcium in cardiac myocytes: calcium transients measured using fluorescence imaging". Society of General Physiologists Series 42: 201–214. 1987-01-01. PMID 3505361.
- ↑ "Mitochondrial free Ca²⁺ levels and their effects on energy metabolism in Drosophila motor nerve terminals". Biophysical Journal 104 (11): 2353–2361. June 2013. doi:10.1016/j.bpj.2013.03.064. PMID 23746507. Bibcode: 2013BpJ...104.2353I.
- ↑ "A technical review of optical mapping of intracellular calcium within myocardial tissue". American Journal of Physiology. Heart and Circulatory Physiology 310 (11): H1388–H1401. June 2016. doi:10.1152/ajpheart.00665.2015. PMID 27016580.
- ↑ "Fluorescence changes of genetic calcium indicators and OGB-1 correlated with neural activity and calcium in vivo and in vitro". The Journal of Neuroscience 28 (29): 7399–7411. July 2008. doi:10.1523/JNEUROSCI.1038-08.2008. PMID 18632944.
- ↑ "Role of calcium in the regulation of mechanical power in insect flight". Proceedings of the National Academy of Sciences of the United States of America 103 (11): 4311–4315. March 2006. doi:10.1073/pnas.0510109103. PMID 16537527. Bibcode: 2006PNAS..103.4311G.
- ↑ "Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans.". Neuron 26 (3): 583–59. June 2000. doi:10.1016/S0896-6273(00)81196-4. PMID 10896155.
- ↑ "Intensiometric biosensors visualize the activity of multiple small GTPases in vivo". Nature Communications 10 (1): 211. January 2019. doi:10.1038/s41467-018-08217-3. PMID 30643148. Bibcode: 2019NatCo..10..211K.
- ↑ "All-Optical Assay to Study Biological Neural Networks". Frontiers in Neuroscience 12: 451. 2018. doi:10.3389/fnins.2018.00451. PMID 30026684.
- ↑ "In vivo odourant response properties of migrating adult-born neurons in the mouse olfactory bulb". Nature Communications 6: 6349. February 2015. doi:10.1038/ncomms7349. PMID 25695931. Bibcode: 2015NatCo...6.6349K.
- ↑ "Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator". Nature Medicine 19 (6): 778–783. June 2013. doi:10.1038/nm.3180. PMID 23685843.
- ↑ "Monitoring Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes with Genetically Encoded Calcium and Voltage Fluorescent Reporters". Stem Cell Reports 5 (4): 582–596. October 2015. doi:10.1016/j.stemcr.2015.08.009. PMID 26372632.
- ↑ "Circular permutation and receptor insertion within green fluorescent proteins". Proceedings of the National Academy of Sciences of the United States of America 96 (20): 11241–11246. September 1999. doi:10.1073/pnas.96.20.11241. PMID 10500161. Bibcode: 1999PNAS...9611241B.
- ↑ 15.0 15.1 "Quantitative Measurement of Ca2+ and Zn2+ in Mammalian Cells Using Genetically Encoded Fluorescent Biosensors". Fluorescent Protein-Based Biosensors. Methods in Molecular Biology. 1071. 2014. pp. 29–47. doi:10.1007/978-1-62703-622-1_3. ISBN 978-1-62703-621-4.
- ↑ "The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins". Trends in Biochemical Sciences 42 (2): 111–129. February 2017. doi:10.1016/j.tibs.2016.09.010. PMID 27814948.
- ↑ 17.0 17.1 "A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein". Nature Biotechnology 19 (2): 137–141. February 2001. doi:10.1038/84397. PMID 11175727.
- ↑ 18.0 18.1 18.2 "Genetically encoded Ca(2+) indicators: properties and evaluation". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1833 (7): 1787–1797. July 2013. doi:10.1016/j.bbamcr.2013.01.011. PMID 23352808.
- ↑ "Dynamic and quantitative Ca2+ measurements using improved cameleons". Proceedings of the National Academy of Sciences of the United States of America 96 (5): 2135–2140. March 1999. doi:10.1073/pnas.96.5.2135. PMID 10051607. Bibcode: 1999PNAS...96.2135M.
- ↑ "A genetically encoded near-infrared fluorescent calcium ion indicator". Nature Methods 16 (2): 171–174. February 2019. doi:10.1038/s41592-018-0294-6. PMID 30664778.
- ↑ "A near-infrared genetically encoded calcium indicator for in vivo imaging". Nature Biotechnology 39 (3): 368–377. March 2021. doi:10.1038/s41587-020-0710-1. PMID 33106681.
- ↑ "Structural basis for photo-induced protein cleavage and green-to-red conversion of fluorescent protein EosFP". Proceedings of the National Academy of Sciences of the United States of America 102 (26): 9156–9159. June 2005. doi:10.1073/pnas.0501874102. PMID 15964985. Bibcode: 2005PNAS..102.9156N.
- ↑ 23.0 23.1 "Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators". Science 347 (6223): 755–760. February 2015. doi:10.1126/science.1260922. PMID 25678659.
- ↑ "Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level". Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7260–7265. June 2000. doi:10.1073/pnas.97.13.7260. PMID 10860991. Bibcode: 2000PNAS...97.7260B.
- ↑ Recombinant obelin: cloning and expression of cDNA purification, and characterization as a calcium indicator. Methods in Enzymology. 305. 2000. pp. 223–249. doi:10.1016/s0076-6879(00)05491-4. ISBN 9780121822064.
- ↑ Oh, J., Lee, C., & Kaang, B. K. (2019). Imaging and analysis of genetically encoded calcium indicators linking neural circuits and behaviors. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology, 23(4), 237–249. https://doi.org/10.4196/kjpp.2019.23.4.237
- ↑ "Optimizing 3D multiphoton fluorescence microscopy". Optics Letters 38 (19): 3945–3948. October 2013. doi:10.1364/OL.38.003945. PMID 24081095. Bibcode: 2013OptL...38.3945K.
- ↑ "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature 388 (6645): 882–887. August 1997. doi:10.1038/42264. PMID 9278050. Bibcode: 1997Natur.388..882M.
- ↑ "Circularly permuted green fluorescent proteins engineered to sense Ca2+". Proceedings of the National Academy of Sciences of the United States of America 98 (6): 3197–3202. March 2001. doi:10.1073/pnas.051636098. PMID 11248055. Bibcode: 2001PNAS...98.3197N.
- ↑ "High-performance calcium sensors for imaging activity in neuronal populations and microcompartments". Nature Methods 16 (7): 649–657. July 2019. doi:10.1038/s41592-019-0435-6. PMID 31209382.
- ↑ 31.0 31.1 "Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein". The Journal of Biological Chemistry 279 (14): 14280–14286. April 2004. doi:10.1074/jbc.M312751200. PMID 14742421.
- ↑ "A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change". Biophysical Journal 90 (5): 1790–1796. March 2006. doi:10.1529/biophysj.105.073536. PMID 16339891. Bibcode: 2006BpJ....90.1790M.
- ↑ "A genetically encoded calcium indicator for chronic in vivo two-photon imaging". Nature Methods 5 (9): 805–811. September 2008. doi:10.1038/nmeth.1243. PMID 19160515.
- ↑ "Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes". Nature Methods 11 (2): 175–182. February 2014. doi:10.1038/nmeth.2773. PMID 24390440.
- ↑ "Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics". Frontiers in Molecular Neuroscience 6: 2. 2013. doi:10.3389/fnmol.2013.00002. PMID 23459413.
- ↑ "Sensitive red protein calcium indicators for imaging neural activity". eLife 5: e12727. March 2016. doi:10.7554/eLife.12727. PMID 27011354.
- ↑ "An expanded palette of genetically encoded Ca²⁺ indicators". Science 333 (6051): 1888–1891. September 2011. doi:10.1126/science.1208592. PMID 21903779. Bibcode: 2011Sci...333.1888Z.
- ↑ "Improved methods for marking active neuron populations". Nature Communications 9 (1): 4440. October 2018. doi:10.1038/s41467-018-06935-2. PMID 30361563. Bibcode: 2018NatCo...9.4440M.
- ↑ "Freeze-frame imaging of synaptic activity using SynTagMA". Nature Communications 11 (1): 2464. May 2020. doi:10.1038/s41467-020-16315-4. PMID 32424147. Bibcode: 2020NatCo..11.2464P.
- ↑ "Freeze-Frame Imaging of Dendritic Calcium Signals With TubuTag" (in English). Frontiers in Molecular Neuroscience 14: 635820. 2021. doi:10.3389/fnmol.2021.635820. PMID 33762909.
- ↑ "Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans". Proceedings of the National Academy of Sciences of the United States of America 113 (8): E1074–E1081. February 2016. doi:10.1073/pnas.1507110112. PMID 26712014. Bibcode: 2016PNAS..113E1074N.
- ↑ "Compressive light-field microscopy for 3D neural activity recording.". Optica 3 (5): 517–24. May 2016. doi:10.1364/optica.3.000517. Bibcode: 2016Optic...3..517P.
- ↑ "A selected review of recent advances in the study of neuronal circuits using fiber photometry". Pharmacology, Biochemistry, and Behavior 201: 173113. February 2021. doi:10.1016/j.pbb.2021.173113. PMID 33444597.
- ↑ "High-density multi-fiber photometry for studying large-scale brain circuit dynamics". Nature Methods 16 (6): 553–560. June 2019. doi:10.1038/s41592-019-0400-4. PMID 31086339. https://www.zora.uzh.ch/id/eprint/184956/1/s41592-019-0400-4.pdf.
- ↑ "Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses". Nature Protocols 11 (3): 566–597. March 2016. doi:10.1038/nprot.2016.021. PMID 26914316.
- ↑ "In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy". Journal of Neurophysiology 92 (5): 3121–3133. November 2004. doi:10.1152/jn.00234.2004. PMID 15128753.
- ↑ "Principles of two-photon excitation microscopy and its applications to neuroscience". Neuron 50 (6): 823–839. June 2006. doi:10.1016/j.neuron.2006.05.019. PMID 16772166.
Further reading
- "A Practical guide to the study of calcium in living cells". Boston: Academic Press. 1994. ISBN 978-0-12-564141-8. http://www.sciencedirect.com/science/bookseries/0091679X/40.
 |