Biology:Calmodulin
| Calmodulin | |
|---|---|
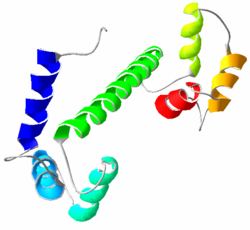 | |
| Identifiers | |
| Symbol | CaM |
| PDB | 1OSA |
| UniProt | P62158 |
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells.[1] It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.[2][3][4]
Structure
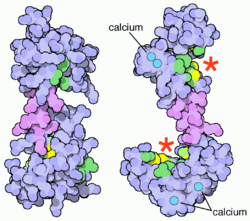
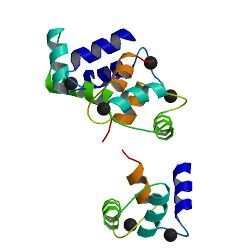
Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand motifs[5] separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain.[6] In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a compact orientation, and the central linker is disordered;[5][6][7][8] in the Ca2+-saturated state, the EF-hand helices adopt an open orientation roughly perpendicular to one another, and the central linker forms an extended alpha-helix in the crystal structure,[5][6] but remains largely disordered in solution.[9] The C-domain has a higher binding affinity for Ca2+ than the N-domain.[10][11]
Calmodulin is structurally quite similar to troponin C, another Ca2+-binding protein containing four EF-hand motifs.[5][12] However, troponin C contains an additional alpha-helix at its N-terminus, and is constitutively bound to its target, troponin I. It therefore does not exhibit the same diversity of target recognition as does calmodulin.
Importance of flexibility in calmodulin
Calmodulin's ability to recognize a tremendous range of target proteins is due in large part to its structural flexibility.[13] In addition to the flexibility of the central linker domain, the N- and C-domains undergo open-closed conformational cycling in the Ca2+-bound state.[9] Calmodulin also exhibits great structural variability, and undergoes considerable conformational fluctuations, when bound to targets.[14][15][16] Moreover, the predominantly hydrophobic nature of binding between calmodulin and most of its targets allows for recognition of a broad range of target protein sequences.[14][17] Together, these features allow calmodulin to recognize some 300 target proteins[18] exhibiting a variety of CaM-binding sequence motifs.
Mechanism
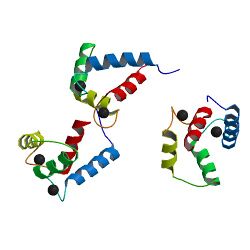
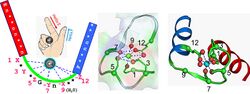
Binding of Ca2+ by the EF-hands causes an opening of the N- and C-domains, which exposes hydrophobic target-binding surfaces.[6] These surfaces interact with complementary nonpolar segments on target proteins, typically consisting of groups of bulky hydrophobic amino acids separated by 10–16 polar and/or basic amino acids.[18][14] The flexible central domain of calmodulin allows the protein to wrap around its target, although alternate modes of binding are known. "Canonical" targets of calmodulin, such as myosin light-chain kinases and CaMKII, bind only to the Ca2+-bound protein, whereas some proteins, such as NaV channels and IQ-motif proteins, also bind to calmodulin in the absence of Ca2+.[14] Binding of calmodulin induces conformational rearrangements in the target protein via "mutually induced fit",[19] leading to changes in the target protein's function.
Calcium binding by calmodulin exhibits considerable cooperativity,[5][11] making calmodulin an unusual example of a monomeric (single-chain) cooperative binding protein. Furthermore, target binding alters the binding affinity of calmodulin toward Ca2+ ions,[20][21][22] which allows for complex allosteric interplay between Ca2+ and target binding interactions.[23] This influence of target binding on Ca2+ affinity is believed to allow for Ca2+ activation of proteins that are constitutively bound to calmodulin, such as small-conductance Ca2+-activated potassium (SK) channels.[24]
Although calmodulin principally operates as a Ca2+ binding protein, it also coordinates other metal ions. For example, in the presence of typical intracellular concentrations of Mg2+ (0.5–1.0 mM) and resting concentrations of Ca2+ (100 nM), calmodulin's Ca2+ binding sites are at least partially saturated by Mg2+.[25] This Mg2+ is displaced by the higher concentrations of Ca2+ generated by signaling events. Similarly, Ca2+ may itself be displaced by other metal ions, such as the trivalent lanthanides, that associate with calmodulin's binding pockets even more strongly than Ca2+.[26][27] Though such ions distort calmodulin's structure[28][29] and are generally not physiologically relevant due to their scarcity in vivo, they have nonetheless seen wide scientific use as reporters of calmodulin structure and function.[30][31][26]
Role in animals
Calmodulin mediates many crucial processes such as inflammation, metabolism, apoptosis, smooth muscle contraction, intracellular movement, short-term and long-term memory, and the immune response.[32][33] Calcium participates in an intracellular signaling system by acting as a diffusible second messenger to the initial stimuli. It does this by binding various targets in the cell including a large number of enzymes, ion channels, aquaporins and other proteins.[4] Calmodulin is expressed in many cell types and can have different subcellular locations, including the cytoplasm, within organelles, or associated with the plasma or organelle membranes, but it is always found intracellularly.[33] Many of the proteins that calmodulin binds are unable to bind calcium themselves, and use calmodulin as a calcium sensor and signal transducer. Calmodulin can also make use of the calcium stores in the endoplasmic reticulum, and the sarcoplasmic reticulum. Calmodulin can undergo post-translational modifications, such as phosphorylation, acetylation, methylation and proteolytic cleavage, each of which has potential to modulate its actions.
Specific examples
Role in smooth muscle contraction
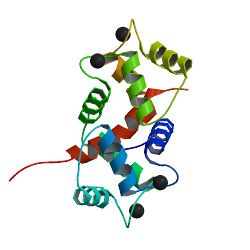
Calmodulin plays an important role in excitation contraction (EC) coupling and the initiation of the cross-bridge cycling in smooth muscle, ultimately causing smooth muscle contraction.[34] In order to activate contraction of smooth muscle, the head of the myosin light chain must be phosphorylated. This phosphorylation is done by myosin light chain (MLC) kinase. This MLC kinase is activated by a calmodulin when it is bound by calcium, thus making smooth muscle contraction dependent on the presence of calcium, through the binding of calmodulin and activation of MLC kinase.[34]
Another way that calmodulin affects muscle contraction is by controlling the movement of Ca2+ across both the cell and sarcoplasmic reticulum membranes. The Ca2+ channels, such as the ryanodine receptor of the sarcoplasmic reticulum, can be inhibited by calmodulin bound to calcium, thus affecting the overall levels of calcium in the cell.[35] Calcium pumps take calcium out of the cytoplasm or store it in the endoplasmic reticulum and this control helps regulate many downstream processes.
This is a very important function of calmodulin because it indirectly plays a role in every physiological process that is affected by smooth muscle contraction such as digestion and contraction of arteries (which helps distribute blood and regulate blood pressure).[36]
Role in metabolism
Calmodulin plays an important role in the activation of phosphorylase kinase, which ultimately leads to glucose being cleaved from glycogen by glycogen phosphorylase.[37]
Calmodulin also plays an important role in lipid metabolism by affecting calcitonin. Calcitonin is a polypeptide hormone that lowers blood Ca2+ levels and activates G protein cascades that leads to the generation of cAMP. The actions of calcitonin can be blocked by inhibiting the actions of calmodulin, suggesting that calmodulin plays a crucial role in the activation of calcitonin.[37]
Role in short-term and long-term memory
Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a crucial role in a type of synaptic plasticity known as long-term potentiation (LTP) which requires the presence of calcium/calmodulin. CaMKII contributes to the phosphorylation of an AMPA receptor which increases the sensitivity of AMPA receptors.[38] Furthermore, research shows that inhibiting CaMKII interferes with LTP.[38]
Role in plants

While yeasts have only a single CaM gene, plants and vertebrates contain an evolutionarily conserved form of CaM genes. The difference between plants and animals in Ca2+ signaling is that the plants contain an extended family of the CaM in addition to the evolutionarily conserved form.[39] Calmodulins play an essential role in plant development and adaptation to environmental stimuli.
Calcium plays a key role in the structural integrity of the cell wall and the membrane system of the cell. However, high calcium levels can be toxic to a plant's cellular energy metabolism and, hence, the Ca2+ concentration in the cytosol is maintained at a submicromolar level by removing the cytosolic Ca2+ to either the apoplast or the lumen of the intracellular organelles. Ca2+ pulses created due to increased influx and efflux act as cellular signals in response to external stimuli such as hormones, light, gravity, abiotic stress factors and also interactions with pathogens.[40]
CMLs (CaM-related proteins)
Plants contain CaM-related proteins (CMLs) apart from the typical CaM proteins. The CMLs have about 15% amino acid similarity with the typical CaMs. Arabidopsis thaliana contains about 50 different CML genes which leads to the question of what purpose these diverse ranges of proteins serve in the cellular function. All plant species exhibit this diversity in the CML genes. The different CaMs and CMLs differ in their affinity to bind and activate the CaM-regulated enzymes in vivo. The CaM or CMLs are also found to be located in different organelle compartments.
Plant growth and development
In Arabidopsis, the protein DWF1 plays an enzymatic role in the biosynthesis of brassinosteroids, steroid hormones in plants that are required for growth. An interaction occurs between CaM and DWF1,[clarification needed] and DWF1 being unable to bind CaM is unable to produce a regular growth phenotype in plants. Hence, CaM is essential for the DWF1 function in plant growth.
CaM binding proteins are also known to regulate reproductive development in plants. For instance, the CaM-binding protein kinase in tobacco acts as a negative regulator of flowering. However, these CaM-binding protein kinase are also present in the shoot apical meristem of tobacco and a high concentration of these kinases in the meristem causes a delayed transition to flowering in the plant.
S-locus receptor kinase (SRK) is another protein kinase that interacts with CaM. SRK is involved in the self-incompatibility responses involved in pollen-pistil interactions in Brassica.
CaM targets in Arabidopsis are also involved in pollen development and fertilization. Ca2+ transporters are essential for pollen tube growth. Hence, a constant Ca2+ gradient is maintained at the apex of pollen tube for elongation during the process of fertilization. Similarly, CaM is also essential at the pollen tube apex, where its primarily role involves the guidance of the pollen tube growth.
Interaction with microbes
Nodule formation
Ca2+ plays an important role in nodule formation in legumes. Nitrogen is an essential element required in plants and many legumes, unable to fix nitrogen independently, pair symbiotically with nitrogen-fixing bacteria that reduce nitrogen to ammonia. This legume-Rhizobium interaction establishment requires the Nod factor that is produced by the Rhizobium bacteria. The Nod factor is recognized by the root hair cells that are involved in the nodule formation in legumes. Ca2+ responses of varied nature are characterized to be involved in the Nod factor recognition. There is a Ca2+ flux at the tip of the root hair initially followed by repetitive oscillation of Ca2+ in the cytosol and also Ca2+ spike occurs around the nucleus. DMI3, an essential gene for Nod factor signaling functions downstream of the Ca2+ spiking signature, might be recognizing the Ca2+ signature. Further, several CaM and CML genes in Medicago and Lotus are expressed in nodules.
Pathogen defense
Among the diverse range of defense strategies plants utilize against pathogens, Ca2+ signaling is very common. Free Ca2+ levels in the cytoplasm increases in response to a pathogenic infection. Ca2+ signatures of this nature usually activate the plant defense system by inducing defense-related genes and the hypersensitive cell death. CaMs, CMLs and CaM-binding proteins are some of the recently identified elements of the plant defense signaling pathways. Several CML genes in tobacco, bean and tomato are responsive to pathogens. CML43 is a CaM-related protein that, as isolated from APR134 gene in the disease-resistant leaves of Arabidopsis for gene expression analysis, is rapidly induced when the leaves are inoculated with Pseudomonas syringae. These genes are also found in tomatoes (Solanum lycopersicum). The CML43 from the APR134 also binds to Ca2+ ions in vitro which shows that CML43 and APR134 are, hence, involved in the Ca2+-dependent signaling during the plant immune response to bacterial pathogens.[41] The CML9 expression in Arabidopsis thaliana is rapidly induced by phytopathogenic bacteria, flagellin and salicylic acid.[42] Expression of soybean SCaM4 and SCaM5 in transgenic tobacco and Arabidopsis causes an activation of genes related to pathogen resistance and also results in enhanced resistance to a wide spectrum of pathogen infection. The same is not true for soybean SCaM1 and SCaM2 that are highly conserved CaM isoforms. The AtBAG6 protein is a CaM-binding protein that binds to CaM only in the absence of Ca2+ and not in the presence of it. AtBAG6 is responsible for the hypersensitive response of programmed cell death in order to prevent the spread of pathogen infection or to restrict pathogen growth. Mutations in the CaM binding proteins can lead to severe effects on the defense response of the plants towards pathogen infections. Cyclic nucleotide-gated channels (CNGCs) are functional protein channels in the plasma membrane that have overlapping CaM binding sites transport divalent cations such as Ca2+. However, the exact role of the positioning of the CNGCs in this pathway for plant defense is still unclear.
Abiotic stress response in plants
Change in intracellular Ca2+ levels is used as a signature for diverse responses towards mechanical stimuli, osmotic and salt treatments, and cold and heat shocks. Different root cell types show a different Ca2+ response to osmotic and salt stresses and this implies the cellular specificities of Ca2+ patterns. In response to external stress CaM activates glutamate decarboxylase (GAD) that catalyzes the conversion of L-glutamate to GABA. A tight control on the GABA synthesis is important for plant development and, hence, increased GABA levels can essentially affect plant development. Therefore, external stress can affect plant growth and development and CaM are involved in that pathway controlling this effect.[citation needed]
Plant examples
Sorghum
The plant sorghum is well established model organism and can adapt in hot and dry environments. For this reason, it is used as a model to study calmodulin's role in plants.[43] Sorghum contains seedlings that express a glycine-rich RNA-binding protein, SbGRBP. This particular protein can be modulated by using heat as a stressor. Its unique location in the cell nucleus and cytosol demonstrates interaction with calmodulin that requires the use of Ca2+.[44] By exposing the plant to versatile stress conditions, it can cause different proteins that enable the plant cells to tolerate environmental changes to become repressed. These modulated stress proteins are shown to interact with CaM. The CaMBP genes expressed in the sorghum are depicted as a “model crop” for researching the tolerance to heat and drought stress.
Arabidopsis
In an Arabidopsis thaliana study, hundreds of different proteins demonstrated the possibility to bind to CaM in plants.[43]
Family members
- Calmodulin 1 (CALM1)
- Calmodulin 2 (CALM2)
- Calmodulin 3 (CALM3)
- calmodulin 1 pseudogene 1 (CALM1P1)
- Calmodulin-like 3 (CALML3)
- Calmodulin-like 4 (CALML4)
- Calmodulin-like 5 (CALML5)
- Calmodulin-like 6 (CALML6)
Other calcium-binding proteins
Calmodulin belongs to one of the two main groups of calcium-binding proteins, called EF hand proteins. The other group, called annexins, bind calcium and phospholipids such as lipocortin. Many other proteins bind calcium, although binding calcium may not be considered their principal function in the cell.
See also
References
- ↑ "Calmodulin: an introduction". Canadian Journal of Biochemistry and Cell Biology 61 (8): 906–10. August 1983. doi:10.1139/o83-115. PMID 6313166.
- ↑ "Calmodulin: a prototypical calcium sensor". Trends in Cell Biology 10 (8): 322–8. August 2000. doi:10.1016/S0962-8924(00)01800-6. PMID 10884684.
- ↑ Neuroscience. Massachusetts: Sinauer Associates. 2012. pp. 95, 147, 148. ISBN 9780878936953.
- ↑ 4.0 4.1 "CALM1 – Calmodulin – Homo sapiens (Human) – CALM1 gene & protein". https://www.uniprot.org/uniprot/P62158.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs". The Biochemical Journal 405 (2): 199–221. July 2007. doi:10.1042/BJ20070255. PMID 17590154.
- ↑ 6.0 6.1 6.2 6.3 "Calmodulin: a prototypical calcium sensor". Trends in Cell Biology 10 (8): 322–8. August 2000. doi:10.1016/s0962-8924(00)01800-6. PMID 10884684.
- ↑ "Solution structure of calcium-free calmodulin". Nature Structural Biology 2 (9): 768–76. September 1995. doi:10.1038/nsb0995-768. PMID 7552748.
- ↑ "Calcium-induced conformational transition revealed by the solution structure of apo calmodulin". Nature Structural Biology 2 (9): 758–67. September 1995. doi:10.1038/nsb0995-758. PMID 7552747.
- ↑ 9.0 9.1 "Solution structure of Ca(2+)-calmodulin reveals flexible hand-like properties of its domains". Nature Structural Biology 8 (11): 990–7. November 2001. doi:10.1038/nsb1101-990. PMID 11685248.
- ↑ "Obtaining site-specific calcium-binding affinities of calmodulin". Protein and Peptide Letters 10 (4): 331–45. August 2003. doi:10.2174/0929866033478852. PMID 14529487.
- ↑ 11.0 11.1 "Calcium binding to calmodulin and its globular domains". The Journal of Biological Chemistry 266 (13): 8050–4. May 1991. doi:10.1016/S0021-9258(18)92938-8. PMID 1902469.
- ↑ "Structures of four Ca2+-bound troponin C at 2.0 A resolution: further insights into the Ca2+-switch in the calmodulin superfamily". Structure 5 (12): 1695–711. December 1997. doi:10.1016/s0969-2126(97)00315-8. PMID 9438870.
- ↑ "Calmodulin's flexibility allows for promiscuity in its interactions with target proteins and peptides". Molecular Biotechnology 27 (1): 33–57. May 2004. doi:10.1385/MB:27:1:33. PMID 15122046.
- ↑ 14.0 14.1 14.2 14.3 "Structural diversity of calmodulin binding to its target sites". The FEBS Journal 280 (21): 5551–65. November 2013. doi:10.1111/febs.12296. PMID 23601118.
- ↑ "Conformational entropy in molecular recognition by proteins". Nature 448 (7151): 325–9. July 2007. doi:10.1038/nature05959. PMID 17637663. Bibcode: 2007Natur.448..325F.
- ↑ "A coupled equilibrium shift mechanism in calmodulin-mediated signal transduction". Structure 16 (5): 736–46. May 2008. doi:10.1016/j.str.2008.02.017. PMID 18462678.
- ↑ "Protein-peptide interaction studies demonstrate the versatility of calmodulin target protein binding". Protein and Peptide Letters 13 (5): 455–65. 2006. doi:10.2174/092986606776819600. PMID 16800798.
- ↑ 18.0 18.1 "Calmodulin Target Database". http://calcium.uhnres.utoronto.ca/ctdb/.
- ↑ "Protein recognition and selection through conformational and mutually induced fit". Proceedings of the National Academy of Sciences of the United States of America 110 (51): 20545–50. December 2013. doi:10.1073/pnas.1312788110. PMID 24297894. Bibcode: 2013PNAS..11020545W.
- ↑ "Effects of myosin light chain kinase and peptides on Ca2+ exchange with the N- and C-terminal Ca2+ binding sites of calmodulin". The Journal of Biological Chemistry 271 (2): 761–7. January 1996. doi:10.1074/jbc.271.2.761. PMID 8557684.
- ↑ "Target recognition by calmodulin: dissecting the kinetics and affinity of interaction using short peptide sequences". Protein Science 5 (7): 1215–28. July 1996. doi:10.1002/pro.5560050701. PMID 8819155.
- ↑ "The neuronal voltage-dependent sodium channel type II IQ motif lowers the calcium affinity of the C-domain of calmodulin". Biochemistry 47 (1): 112–23. January 2008. doi:10.1021/bi7013129. PMID 18067319.
- ↑ "An allosteric model of calmodulin explains differential activation of PP2B and CaMKII". Proceedings of the National Academy of Sciences of the United States of America 105 (31): 10768–73. August 2008. doi:10.1073/pnas.0804672105. PMID 18669651. Bibcode: 2008PNAS..10510768S.
- ↑ "Structural basis for calmodulin as a dynamic calcium sensor". Structure 20 (5): 911–23. May 2012. doi:10.1016/j.str.2012.03.019. PMID 22579256.
- ↑ "Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1813 (5): 913–21. May 2011. doi:10.1016/j.bbamcr.2011.01.017. PMID 21262274.
- ↑ 26.0 26.1 "Terbium (III) emission as a probe of calcium(II) binding sites in proteins". Journal of the American Chemical Society 98 (25): 8255–60. December 1976. doi:10.1021/ja00441a060. PMID 993525.
- ↑ "Terbium as luminescent probe of calmodulin calcium-binding sites; domains I and II contain the high-affinity sites". FEBS Letters 116 (2): 269–72. July 1980. doi:10.1016/0014-5793(80)80660-0. PMID 7409149.
- ↑ "Coordination to lanthanide ions distorts binding site conformation in calmodulin". Proceedings of the National Academy of Sciences of the United States of America 115 (14): E3126–E3134. April 2018. doi:10.1073/pnas.1722042115. PMID 29545272. Bibcode: 2018PNAS..115E3126E.
- ↑ "Activation of calmodulin by various metal cations as a function of ionic radius". Molecular Pharmacology 26 (1): 75–82. July 1984. PMID 6087119. https://molpharm.aspetjournals.org/content/26/1/75.
- ↑ "Lanthanide ion luminescence probes of the structure of biological macromolecules". Accounts of Chemical Research 14 (12): 384–392. 1981-12-01. doi:10.1021/ar00072a004. ISSN 0001-4842.
- ↑ "Characterization of lanthanide (III) ion binding to calmodulin using luminescence spectroscopy". Biochemistry 24 (23): 6639–45. November 1985. doi:10.1021/bi00344a051. PMID 4084548.
- ↑ "Home Page for Calmodulin". http://structbio.vanderbilt.edu/cabp_database/general/prot_pages/calmod.html#func.
- ↑ 33.0 33.1 "Calmodulin". https://www.ebi.ac.uk/interpro/potm/2003_3/Page_1.htm.
- ↑ 34.0 34.1 "Ca(2+)-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells". The Journal of Biological Chemistry 269 (13): 9912–20. April 1994. doi:10.1016/S0021-9258(17)36969-7. PMID 8144585. http://www.jbc.org/content/269/13/9912.
- ↑ "Calmodulin and the regulation of smooth muscle contraction". Molecular and Cellular Biochemistry 135 (1): 21–41. June 1994. doi:10.1007/bf00925958. PMID 7816054.
- ↑ "Regulation of calcium channels in smooth muscle: new insights into the role of myosin light chain kinase". Channels 8 (5): 402–13. 2014-10-31. doi:10.4161/19336950.2014.950537. PMID 25483583.
- ↑ 37.0 37.1 "Calcium/calmodulin-mediated action of calcitonin on lipid metabolism in rats". The Journal of Clinical Investigation 82 (4): 1165–72. October 1988. doi:10.1172/jci113713. PMID 2844851.
- ↑ 38.0 38.1 "Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism". Proceedings of the National Academy of Sciences of the United States of America 92 (24): 11175–9. November 1995. doi:10.1073/pnas.92.24.11175. PMID 7479960. Bibcode: 1995PNAS...9211175L.
- ↑ "Plant calmodulins and calmodulin-related proteins: multifaceted relays to decode calcium signals". Plant Signaling & Behavior 1 (3): 96–104. May 2006. doi:10.4161/psb.1.3.2998. PMID 19521489.
- ↑ Virdi, Amardeep S.; Singh, Supreet; Singh, Prabhjeet (2015). "Abiotic stress responses in plants: roles of calmodulin-regulated proteins". Frontiers in Plant Science 6: 809. doi:10.3389/fpls.2015.00809. ISSN 1664-462X. PMID 26528296.
- ↑ "Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. tomato". Plant Molecular Biology 58 (6): 887–897. August 2005. doi:10.1007/s11103-005-8395-x. PMID 16240180.
- ↑ "CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway". The Plant Journal 71 (6): 976–89. September 2012. doi:10.1111/j.1365-313x.2012.05045.x. PMID 22563930.
- ↑ 43.0 43.1 "Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench)". Plant Molecular Biology 48 (5–6): 713–26. 2002. doi:10.1023/a:1014894130270. PMID 11999845.
- ↑ "A temperature-responsive gene in sorghum encodes a glycine-rich protein that interacts with calmodulin". Biochimie 137 (Supplement C): 115–123. June 2017. doi:10.1016/j.biochi.2017.03.010. PMID 28322928.
External links
- "Myelin-associated Glycoprotein". Molecule of the Month. RCSB PDB. July 2020. http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb44_1.html.
- "Home Page for Calmodulin". EF-Hand Calcium-Binding Proteins Data Library. Vanderbilt University. http://structbio.vanderbilt.edu/cabp_database/general/prot_pages/calmod.html.
- Ikura, Mitsuhiko (2000). "Calmodulin Target Database". Journal of Structural and Functional Genomics (Ontario Cancer Institute, University of Toronto) 1 (1): 8–14. doi:10.1023/a:1011320027914. PMID 12836676. http://calcium.uhnres.utoronto.ca/ctdb/ctdb/home.html. Retrieved 2008-03-22.
- Calmodulin at the US National Library of Medicine Medical Subject Headings (MeSH)
- InterPro: IPR015754
- Proteopedia page for Calmodulin and its conformational change
 |
