Biology:Hamamelidaceae
| Hamamelidaceae | |
|---|---|

| |
| Fothergilla major (Witch alder) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Order: | Saxifragales |
| Family: | Hamamelidaceae R.Br.[1][2] |
| Type genus | |
| Hamamelis L.
| |
| Subfamilies | |
|
See text | |
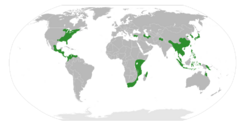
| |
| The range of Hamamelidaceae. | |
Hamamelidaceae, commonly referred to as the witch-hazel family, is a family of flowering plants in the order Saxifragales. The clade consists of shrubs and small trees positioned within the woody clade of the core Saxifragales. An earlier system, the Cronquist system, recognized Hamamelidaceae in the Hamamelidales order.
Description
The Hamamelidaceae are distinguishable from other families in the Saxifragales due to the range of floral characteristics that are generally uniform though all genera. Uniform characteristics include stipules borne on stems with leaves often 2-ranked.[3] Genera usually have a two carpel gynoecium, although some species show variation. Other characteristics include a multicellular stigma, with shallow papillae or ridges.[4]
Anthers
Anther structure and the modes of opening are considered to be one of the most important features in the systematics and evolution of hamamelids. The anthers in Hamamelids are on average shorter than in other families in the Saxifragales.[3] The anther valve openings are unique pleismorphic features that contrast with the simple longitudinal slits of the anthers in the upper Hamamelidae where the pollen is predominantly wind-driven.[5]
The three types of anthers found in the Hamamelidaceae are:
- Type 1) The theca (or sheath of anther) opens like a window with two wings; a common anther type.[5]
- Type 2) There is one valve opening to reveal two pollen sacs. Five genera in the Hamamelidoideae subfamily, confined to the Southern Hemisphere (Trichcladus, Dicoryphe, Ostrearia, Neostrearia, Noahdendron) are known to have this anther type.[5]
- Type 3) One valve opens a wing of anther tissue towards the center of the flower revealing one pollen sac. The two genera, Exbucklandia and Hamamelis is known to have this anther type.[5]
Pollen
Plants of the Hamamelidaceae have sticky pollen, which may have influenced the type of pollination that is seen in this family. Pollination is predominantly via insects or wind. However, the insect-pollinated genus Disanthus has been known to wind-pollinate (although inefficiently) in the event pollinators do not visit its flowers.[6] The genus Rhodoleia is unique because it is bird-pollinated.[5] [3][7]
The pollen structure in the lower Hamamelidae is relatively uniform. The pollen patterns are tricolpate[5][3] with reticulate exines.
Flowers
The petals of the Hamamelidaceae are generally narrow and ribbon-like. The exceptions are the genera Corylopsis and Rhodoleia, which have spathulate or circular-like petals.[5]
The flowers of Hamamelidaceae are mostly bisexual with perianth parts, which mature to fruits arranged in spikes, racemes or nonglobose heads.[5][3]
Breeding systems
The anemophilous groups within the Hamamelidaceae are often andromonoecious. Self-incompatibility is common, but self-compatibility occurs in some genera such as Hamamelis.[5]
Taxonomy
The fossil record dates from the Eocene.[8] Hamamelidaceae was established by Brown in 1818[1] as the Hamamelideae, including four genera.[9] The phylogenetic relationship of the Hamamelidaceae have been revisited several times since the first comprehensive classification of the family in 1930.[10] This was clarified in 1998 by the molecular phylogenetic work of the Angiosperm Phylogeny Group (APG) which placed the family within the eudicot order Saxifragales. In doing so, it separated one of the existing subfamilies, the Altingioideae, which formed the basal group, into its own family within the order, the Altingiaceae.[11]
| Cladogram of Saxifragales families[12][13][2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cynomorium (Cynomoriaceae) remains unplaced within this tree |
Subdivision
Subfamilies
The infrafamilial classification of the Hamamelidaceae has been controversial, and has undergone a number of revisions based on morphology, the best known of which are those of Harms (1930)[10] and Endress (1989).[5][14][15]
Morphological and DNA studies have supported monophyly of the Hamamelidoideae[16][17] and have recognized the separation of the Rhodoleioideae and Disanthoideae subfamily and newly erected Mytilarioideae. [18][14][15] [19] [20] [21]
The relationships between Exbucklandioideae and the other subfamilies have proven controversial. The unresolved monophyly of Exbucklandioideae and the clades of Disanthoideae, Rhodoleioideae, Exbucklandioideae or even Mytilarioideae being a sister clade to Hamamelidoideae may have been a result of differing DNA methodologies researchers have used to produce phylogenetic trees and the inclusion or exclusion of certain genera used as outgroups in their analyses. However, the sister relationship of Disanthoideae and Hamamelidoideae has been well supported,[14][17][15][22] although some researchers[16] do not support this. Strong support for making Altingioideae a family has been recognized by textbooks[3] and the Angiosperm Phylogeny Group. Research continues to resolve the deep relationships of the subfamilies within the Hamamelidaceae by incorporating whole or fragmentary fossil evidence.[18][20]
Hamamelidaceae contains 27-30 genera and 80-140 species distributed among five to six subfamilies. The subfamilies are Exbucklandioideae, Rhodoleioideae, Mytilarioideae, Disanthoideae, Hamamelidoideae, and Altingioideae, which has been elevated to a family Altingiaceae in some recent treatments.[3] Many of the subfamilies are monotypic and the majority of the species lie within the Hamamelidoideae, which has 22 genera.
The long-standing question of whether Altingioideae should be a separate family has been assessed and supported by morphological and molecular phylogenetic studies.[2][23][3][22][24] The resulting subfamilial structure was eventually resolved in a series of molecular studies in the late 1990s, resulting in five distinct subfamilies, the majority of the genera residing in the nominative subfamily, Hamamelidoideae:[9]
- Subfamilies (number of genera)
- Exbucklandioideae (1 genus; Exbucklandia)
- Rhodoleioideae (1 genus; Rhodoleia)
- Mytilarioideae (2 genera; Mytilaria, Chunia)
- Disanthoideae (1 genus; Disanthus)
- Hamamelidoideae (22 genera; see Tribes below)
Tribes
The relatively large size of subfamily Hamamelidoideae and its further subdivision into tribes has also been a matter of study and controversy. Six tribes are now recognized. The revised structure has greatly reduced Hamamelideae to a monotypic taxon, which had previously been further divided into subtribes:[25]
- Corylopsideae (1 genus; Corylopsis)
- Dicorypheae (5 genera; Dicoryphe, Trichocladus, Neostrearia, Noahdendron, Ostrearia)
- Eustigmateae (4 genera; Eustigma, Fortunearia, Sinowilsonia, Molinadendron)
- Fothergilleae (7 genera; Fothergilla, Parrotiopsis, Parrotia, Shaniodendron, Sycopsis, Distylium, Distyliopsis)
- Hamamelideae (1 genus; Hamamelis)
- Loropetaleae (4 genera; Loropetalum (including Tetrathyrium), Maingaya, Embolanthera, Matudaea)
Genera
- Chunia (1 species; Hainan)
- Corylopsis (winter-hazel; about 30 species; east Asia)
- Dicoryphe
- Disanthus (1 species; east Asia)
- Distyliopsis
- Distylium (about 10 species; east Asia, Himalayas)
- Embolanthera
- Eustigma
- Exbucklandia (3 species; Assam, China , southeast Asia)
- Fortunearia (1 species; eastern China )
- Fothergilla (fothergilla; 3 species; southeastern United States )
- Hamamelis (witch-hazel; 4 species; eastern North America, east Asia)
- †Langeria (Wolfe) & Wehr) Eocene 1 species
- Loropetalum (three species; east Asia)
- Maingaya
- Matudaea
- Molinadendron
- Mytilaria
- Neostrearia
- Noahdendron
- Ostrearia
- Parrotia (Persian ironwood; 1 species; Alborz Mountains of southwest Asia)
- Parrotiopsis (1 species; Himalaya )
- Rhodoleia (about 7 species; southeast Asia)
- Sinowilsonia (1 species; western China)
- Sycopsis (about 7 species; southeast Asia)
- Tetrathyrium
- Trichocladus
Distribution and habitat
The Hamamelidaceae were widely distributed in the Northern Hemisphere during the Upper Cretaceous and early Tertiary.[5] [18] Quaternary glaciation across the Northern Hemisphere caused the extinction of numerous species and the restricted distribution of others. Hamamelidaceae were obliterated from Europe along with numerous other genera of plants that were unable to escape the ice sheets due to geography (the Mediterranean Sea and Alps forming barriers that did not exist in North America and Asia)[26]
The largest subfamily, the Hamamelidoideae, is now distributed in North America and western and eastern Asia. The Hamamelidoideae subtribe Dicoryphinae is now restricted to the African (including Madagascar and Comores) and Australia n continents.[26] [4][14] Disanthoideae and Rhodoleioideae are now restricted to southern China and the Caucasus region.[26] Mytilarioideae is restricted to eastern Asia. Altingioideae is now restricted to eastern Asia and western Asia and North America between central Mexico and Belize.[26] [23]
References
- ↑ 1.0 1.1 Brown 1818.
- ↑ 2.0 2.1 2.2 APG IV 2016.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Judd, W.S., Campbell, C.S., Kellogg, E.A., Stevens, P.F. & Donoghue, M.J. 2010. "Plant Systematics: A Phylogenetic Approach, 3rd ed.". In [eds.], Plant Systematics: A Phylogenetic Approach, 3rd ed. In [eds.]. Sinauer Associates, Inc., Massachusetts.
- ↑ 4.0 4.1 Endress, P. K. (1989). "Aspects of evolutionary differentiation of the Hamamelidaceae and the Lower Hamamelididae". Plant Systematics and Evolution 162 (1–4): 193–211. doi:10.1007/BF00936917.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 Endress 1989.
- ↑ Xiao, Y.-A.; Neog, B.; Xiao, Y.-H.; Li, X.-H.; Liu, J.-C.; He, P. (2009). "Pollination biology of Disanthus cercidifolius var. longipes, an endemic and endangered plant in China". Biologia 64 (4): 731–736. doi:10.2478/s11756-009-0122-7.
- ↑ Gu, L.; Z. Luo; D. Zhang; S. S. Renner (2010). "Passerine pollination of Rhodoleia championii (Hamamelidaceae) in subtropical China". Biotropica 42 (3): 336–341. doi:10.1111/j.1744-7429.2009.00585.x.
- ↑ Ludvigsen 2011.
- ↑ 9.0 9.1 Li 1997.
- ↑ 10.0 10.1 Harms 1930.
- ↑ APG I 1998.
- ↑ Jian et al 2008.
- ↑ Stevens 2019.
- ↑ 14.0 14.1 14.2 14.3 Li et al 1999a.
- ↑ 15.0 15.1 15.2 Li et al 1999b.
- ↑ 16.0 16.1 Shi et al 1998.
- ↑ 17.0 17.1 Qiu, Y.-L. (1998). "Phylogenetics of the Hamamelidae and their allies: parsimony analyses of nucleotide sequences of the plastid gene rbcL". International Journal of Plant Sciences 159 (6): 891–905. doi:10.1086/297611.
- ↑ 18.0 18.1 18.2 Magallon, S.; Herendeen, P.S.; Crane, P.R. (2001). "Androdecidua endressii gen. et sp. nov., from the Late Cretaceous of Georgia (United States): Further Floral Diversity in Hamamelidoideae (Hamamelidaceae)". International Journal of Plant Sciences 162 (4): 963–983. doi:10.1086/320770.
- ↑ Chang, H.T. (1979). "Hamamelidaceae". Flora Reipublicae Popularis Sinicae 35: 36–116.
- ↑ 20.0 20.1 Magallon, S. (2007). "From fossils to molecules: Phylogeny and the core eudicot floral groundplan in Hamamelidoideae (Hamamelidaceae, Saxifragales)". Systematic Botany 32 (2): 317–347. doi:10.1600/036364407781179617.
- ↑ Huang, G.L. (1986). "Comparative anatomical studies on the woods of the Hamamelidaceae in China". Sunyatsenia 1: 24–26.
- ↑ 22.0 22.1 Pan, K.-Y.; Lu, A.-M.; Wen, J. (1990). "Characters of Leaf Epidermis in Hamamelidaceae (s. l.).". Sunyatsenia 28: 10–26.
- ↑ 23.0 23.1 Ickert-Bond, S.M.; Pigg, K.B.; Wen, J. (2005). "Comparative infructescence morphology in Liquidambar (Altingiaceae) and its evolutionary significance". American Journal of Botany 92 (8): 1234–1255. doi:10.3732/ajb.92.8.1234. PMID 21646145.
- ↑ Takhtajan, A. 1997. "Diversity and classification of flowering plants". In [eds.], Diversity and classification of flowering plants. Columbia University Press, New York.
- ↑ Li & Bogle 2001.
- ↑ 26.0 26.1 26.2 26.3 Zhi-yun, Z.; An-ming, L. (1995). "Hamamelidaceae: Geographic distribution, fossil history and origin". Acta Phytotaxonomica Sinica 33 (4): 313–339.
Bibliography
Books and theses
- Brown, Robert (1818). Characters and Descriptions of Three New Species of Plants. Extracted from the 'Narrative of a Journey in the Interior of China', by Clarke Abel, Esq., pp. 374–379. Longman, London. https://www.biodiversitylibrary.org/item/116352#page/331/mode/1up.
- Byng, James W. (2014). "Saxifragales". The Flowering Plants Handbook: A practical guide to families and genera of the world. Plant Gateway Ltd.. pp. 156–166. ISBN 978-0-9929993-1-5. https://books.google.com/books?id=yoLaBAAAQBAJ.
- Christenhusz, Maarten J. M.; Fay, Michael F.; Chase, Mark W. (2017). "Saxifragales". Plants of the World: An Illustrated Encyclopedia of Vascular Plants. University of Chicago Press. pp. 231–244. ISBN 978-0-226-52292-0. https://books.google.com/books?id=LLo7DwAAQBAJ.
- Harms, H. (1930). "Hamamelidaceae". in Engler, Adolf. Die Natürlichen Pflanzenfamilien. 18A. Leipzig: Verlag von Wilhelm Engelmann. pp. 330–343.
- Li, Jianhua (December 1997). Systematics of the Hamamelidaceae based on morphological and molecular evidence (PhD thesis). Department of Plant Biology, University of New Hampshire.
- Ludvigsen, Rolf (2011). "Eocene compression floras". Life in Stone: A Natural History of British Columbia's Fossils. UBC Press. p. 243. ISBN 978-0774841511. https://books.google.com/books?id=Jf1bbevG5TMC&pg=PA243.
Articles
- Endress, Peter K. (1989). "A suprageneric taxonomic classification of the Hamamelidaceae". Taxon 38 (3): 371–376. doi:10.2307/1222267.
- Gapinski, Andrew (2014). "Hamamelidaceae, Part 1: Exploring the Witch-hazels of the Arnold Arboretum". Arnoldia 72 (2): 2–17. http://arnoldia.arboretum.harvard.edu/pdf/articles/2014-72-2-hamamelidaceae-part-1-exploring-the-witch-hazels-of-the-arnold-arboretum.pdf.
- Gapinski, Andrew (2015). "Hamamelidaceae, Part 2: Exploring the Witch-hazel Relatives of the Arnold Arboretum". Arnoldia 72 (4): 20–35. http://arnoldia.arboretum.harvard.edu/pdf/articles/2015-72-4-hamamelidaceae-part-2-exploring-the-witch-hazel-relatives-of-the-arnold-arboretum.pdf.
- Jian, Shuguang; Soltis, Pamela S.; Gitzendanner, Matthew A.; Moore, Michael J.; Li, Ruiqi; Hendry, Tory A.; Qiu, Yin-Long; Dhingra, Amit et al. (February 2008). "Resolving an ancient, rapid radiation in Saxifragales". Systematic Biology 57 (1): 38–57. doi:10.1080/10635150801888871. PMID 18275001.
- Li, Jianhua; Bogle, A. Linn; Klein, Anita S. (September 1999). "Phylogenetic relationships in the Hamamelidaceae: Evidence from the nucleotide sequences of the plastid gene matK". Plant Systematics and Evolution 218 (3–4): 205–219. doi:10.1007/BF01089228. https://www.researchgate.net/publication/226359159.
- Li, Jianhua; Bogle, A. Linn; Klein, Anita S. (July 1999). "Phylogenetic relationships of the Hamamelidaceae inferred from sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA". American Journal of Botany 86 (7): 1027–1037. doi:10.2307/2656620. PMID 10406726.
- Li, Jianhua; Bogle, A. Linn (2001). "A new suprageneric classification system of the Hamamelidoideae based on morphology and sequences of nuclear and chloroplast DNA". Harvard Papers in Botany 5 (2): 499–515. ISSN 1043-4534.
- Petruzzello, Melissa (12 September 2017). "Hamamelidaceae". Hamamelidaceae. Encyclopædia Britannica. https://www.britannica.com/plant/Hamamelidaceae.
- Shi, Suhua; Chang, H.T.; Chen, Yueqing; Qu, Lianghu; Wen, Jun (January 1998). "Phylogeny of the Hamamelidaceae based on the ITS sequences of nuclear ribosomal DNA". Biochemical Systematics and Ecology 26 (1): 55–69. doi:10.1016/s0305-1978(97)00075-6.
- APG
- Angiosperm Phylogeny Group (1998), "An ordinal classification for the families of flowering plants", Annals of the Missouri Botanical Garden 85 (4): 531–553, doi:10.2307/2992015, https://www.researchgate.net/publication/279592674
- Angiosperm Phylogeny Group II (2003), Bremer, B., K. Bremer, M.W. Chase, J.L. Reveal, D.E. Soltis, P.S. Soltis & P.F. Stevens, "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II", Botanical Journal of the Linnean Society 141 (4): 399–436, doi:10.1046/j.1095-8339.2003.t01-1-00158.x, http://www.biodiversitas.org.br/listasmg/apg2.pdf, retrieved 2019-11-15
- Angiosperm Phylogeny Group III (2009), Bremer, B., K. Bremer, M.W. Chase, M.F. Fay, J.L. Reveal, D.E. Soltis, P.S. Soltis & P.F. Stevens, "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III", Botanical Journal of the Linnean Society 161 (2): 105–121, doi:10.1111/j.1095-8339.2009.00996.x
- Angiosperm Phylogeny Group IV (2016). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV". Botanical Journal of the Linnean Society 181 (1): 1–20. doi:10.1111/boj.12385.
Websites
- Cole, Theodor C. H.; Hilger, Hartmut H.; Stevens, Peter F. (May 2019), Angiosperm Phylogeny Poster - Flowering Plant Systematics, http://www2.biologie.fu-berlin.de/sysbot/poster/poster1.pdf, retrieved 24 September 2019
- Stevens, P.F. (2019). "Saxifragales". Missouri Botanical Garden. http://www.mobot.org/MOBOT/Research/APweb/orders/saxifragalesweb.htm#Saxifragales. (see also Angiosperm Phylogeny Website)
- Soltis, D; Soltis, P; Arakaki, M (2006). "Saxifragales". http://tolweb.org/Saxifragales.
- WFO (2019). "Saxifragales Bercht. & J.Presl". http://worldfloraonline.org/taxon/wfo-9000000481.
- Hamamelidaceae in L. Watson and M.J. Dallwitz (1992 onwards). The families of flowering plants.
Wikidata ☰ Q148479 entry
 |

