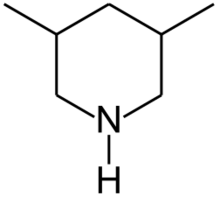
Chemistry:3,5-Dimethylpiperidine
From HandWiki
Revision as of 22:35, 28 June 2021 by imported>AstroAI (url)
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,5-Dimethylpiperidine | |
| Other names
3,5-Lupetidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C7H15N | |
| Molar mass | 113.204 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.853 g/mL (mixture) |
| Boiling point | 144 °C (291 °F; 417 K) |
| Low | |
| Solubility in other solvents | Most organic solvents |
Refractive index (nD)
|
1.4454 |
| Hazards | |
| Main hazards | Flammable |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
| Flash point | 33 °C (91 °F; 306 K) |
| Related compounds | |
Related compounds
|
2,6-Dimethylpiperidine Piperidine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,5-Dimethylpiperidines are chemical compounds with the formula C5H8(CH3)2NH. Two diastereomers exist: the achiral R,S isomer and the chiral R,R/S,S enantiomeric pair. 3,5-Dimethylpiperidine is a precursor to tibric acid.
The compound is typically prepared by hydrogenation of 3,5-dimethylpyridine. Both diastereomers also arise from the reduction of 3,5-dimethylpyridine with lithium triethylborohydride.[1]
References
- ↑ Blough, Bruce E.; Carroll; F. Ivy (1993). "Reduction of isoquinoline and pyridine-containing heterocycles with lithium triethylborohydride (Super-Hydride)". Tetrahedron Letters 34 (45): 7239–42. doi:10.1016/S0040-4039(00)79297-5.
 |

