Chemistry:Aminophosphonate
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CR'2NR"2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups.[1] Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have had medical applications investigated.[2] Phosphonates are more difficult to hydrolyse than phosphates.[3]
Preparation
Aminophosphonates are often prepared by hydrophosphonylation, usually the condensation of imines and phosphorous acid. In the Pudovik reaction or Kabachnik–Fields reaction the esters of phosphorous acid are employed, e.g. diphenylphosphite. Because these compounds are of pharmaceutical interest, methods have been developed to induce these additions asymmetrically.[4][5]
Examples
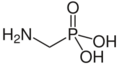
Aminomethylphosphonic acid, the simplest possible aminophosphonate.
Glyphosate, a common though contentious herbicide
EDTMP, a chelating agent. Its 153Sm complex (Quadramet) is used in the treatment of cancer
References
- ↑ Pedro Merino; Eugenia Marqués-López; Raquel P. Herrera (2008). "Catalytic Enantioselective Hydrophosphonylation of Aldehydes and Imines". Advanced Synthesis & Catalysis 350 (9): 1195–1208. doi:10.1002/adsc.200800131.
- ↑ Tušek-Božić, LJ (2013). "Aminophosphonate metal complexes of biomedical potential". Current Medicinal Chemistry 20 (16): 2096–117. doi:10.2174/0929867311320160004. PMID 23432587.
- ↑ Orsini, F; Sello, G; Sisti, M (2010). "Aminophosphonic acids and derivatives. Synthesis and biological applications.". Current Medicinal Chemistry 17 (3): 264–89. doi:10.2174/092986710790149729. PMID 20214568.
- ↑ Foroogh Bahrami; Farhad Panahi; Ali Khalafinezhad (2016). "Synthesis of new α-aminophosphonate derivatives incorporating benzimidazole, theophylline and adenine nucleobases using L-cysteine functionalized magnetic nanoparticles (LCMNP) as magnetic reusable catalyst: evaluation of their anticancer properties". RSC Advances 6 (9): 5915–5924. doi:10.1039/C5RA21419J. Bibcode: 2016RSCAd...6.5915B.
- ↑ Mucha, Artur; Kafarski, Paweł; Berlicki, Łukasz (2011). "Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry". Journal of Medicinal Chemistry 54 (17): 5955–5980. doi:10.1021/jm200587f. PMID 21780776.
 |