Chemistry:Melanocyte-inhibiting factor
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a605038 |
| Routes of administration | IV |
| Pharmacokinetic data | |
| Bioavailability | 100% (injected) |
| Metabolism | plasma protease enzymes |
| Excretion | N/A |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H24N4O3 |
| Molar mass | 284.360 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
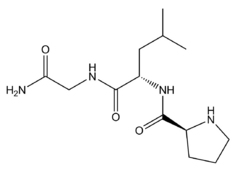
Melanocyte-inhibiting factor (also known as Pro-Leu-Gly-NH2, Melanostatin, MSH release–inhibiting hormone or MIF-1) is an endogenous peptide fragment derived from cleavage of the hormone oxytocin, but having generally different actions in the body.[1][2] MIF-1 produces multiple effects, both blocking the effects of opioid receptor activation,[3][4][5][6][7][8] while at the same time acting as a positive allosteric modulator of the D2 and D4 dopamine receptor subtypes,[9][10][11][12][13][14][15][16][17] as well as inhibiting release of other neuropeptides such as alpha-MSH,[18][19][20] and potentiating melatonin activity.[21]
This complex mix of actions produces a profile of antidepressant,[22][23][24] nootropic,[25][26][27][28] and anti-Parkinsonian effects when MIF-1 is administered,[29][30][31] and it has been investigated for various medical uses. MIF-1 is unusually resistant to metabolism in the bloodstream,[32] and crosses the blood–brain barrier easily,[33][34] though it is poorly active orally and is usually injected. Several other closely related peptides with important actions in the body include Tyr-MIF-1 and endomorphin-1 and -2.[35][36][37][38][39]
See also
- Nemifitide
- Doreptide
References
- ↑ "Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone". Proceedings of the National Academy of Sciences of the United States of America 68 (7): 1428–33. July 1971. doi:10.1073/pnas.68.7.1428. PMID 5283931. Bibcode: 1971PNAS...68.1428C.
- ↑ "Prolyl-leucyl-glycinamide shares some effects with oxytocin but decreases oxytocin levels". Physiology & Behavior 83 (3): 475–81. December 2004. doi:10.1016/j.physbeh.2004.08.034. PMID 15581670.
- ↑ "Antagonism of morphine-induced catalepsy by L-prolyl-L-leucyl-glycinamide". European Journal of Pharmacology 53 (2): 119–25. January 1979. doi:10.1016/0014-2999(79)90156-0. PMID 32058.
- ↑ "Opiate receptor antagonism by L-prolyl-L-leucyl-glycinamide, MIF-I". Peptides 1 (4): 293–9. 1980. doi:10.1016/0196-9781(80)90006-6. PMID 6117839.
- ↑ "Effect of prolyl-leucyl-glycinamide and alpha-melanocyte-stimulating hormone on levorphanol-induced analgesia, tolerance and dependence". Life Sciences 34 (26): 2559–66. June 1984. doi:10.1016/0024-3205(84)90041-9. PMID 6146083.
- ↑ "Antagonism of morphine analgesia by prolyl-leucyl-glycinamide (MIF-1) in humans". Pharmacology Biochemistry and Behavior 21 (6): 975–8. December 1984. doi:10.1016/S0091-3057(84)80083-0. PMID 6151672.
- ↑ "Existence of antiopiate systems as illustrated by MIF-1/Tyr-MIF-1". Life Sciences 39 (23): 2153–9. December 1986. doi:10.1016/0024-3205(86)90391-7. PMID 2878336.
- ↑ "Antiopioid properties of the TYR-MIF-1 family". Methods and Findings in Experimental and Clinical Pharmacology 26 (9): 673–7. November 2004. doi:10.1358/mf.2004.26.9.872564. PMID 15632952.
- ↑ "Effects of L-prolyl-L-leucyl-glycine amide (MIF-I) on dopaminergic neurons". Pharmacology Biochemistry and Behavior 5 (Suppl 1): 125–7. 1976. doi:10.1016/0091-3057(76)90340-3. PMID 13412.
- ↑ "MIF-1: effects on norepinephrine, dopamine and serotonin metabolism in certain discrete brain regions". Pharmacology Biochemistry and Behavior 16 (2): 229–33. February 1982. doi:10.1016/0091-3057(82)90153-8. PMID 6122214.
- ↑ "Mesolimbic and striatal dopamine receptor supersensitivity: prophylactic and reversal effects of L-prolyl-L-leucyl-glycinamide (PLG)". Peptides 6 (2): 179–83. 1985. doi:10.1016/0196-9781(85)90036-1. PMID 2863809.
- ↑ "Mechanism of action of L-leucyl-glycinamide and its effect on Parkinson's disease". Advances in Neurology 45: 587–90. 1987. PMID 2881450.
- ↑ "Modulation of agonist binding to human dopamine receptor subtypes by L-prolyl-L-leucyl-glycinamide and a peptidomimetic analog". The Journal of Pharmacology and Experimental Therapeutics 315 (3): 1228–36. December 2005. doi:10.1124/jpet.105.091256. PMID 16126839. http://pdfs.semanticscholar.org/8f44/e9376f1912434982484dd40a4782134dca91.pdf.
- ↑ "Design and synthesis of photoaffinity-labeling ligands of the L-prolyl-L-leucylglycinamide binding site involved in the allosteric modulation of the dopamine receptor". Journal of Medicinal Chemistry 49 (1): 307–17. January 2006. doi:10.1021/jm050644n. PMID 16392815.
- ↑ "Allosteric modulation of the dopamine receptor by conformationally constrained type VI beta-turn peptidomimetics of Pro-Leu-Gly-NH2". Journal of Medicinal Chemistry 50 (26): 6725–9. December 2007. doi:10.1021/jm070895r. PMID 18052024.
- ↑ "Allosteric modulation of the dopamine D2 receptor by Pro-Leu-Gly-NH2 peptidomimetics constrained in either a polyproline II helix or a type II beta-turn conformation". Journal of Medicinal Chemistry 52 (7): 2043–51. April 2009. doi:10.1021/jm801575w. PMID 19271750.
- ↑ "Specific binding of photoaffinity-labeling peptidomimetics of Pro-Leu-Gly-NH2 to the dopamine D2L receptor: evidence for the allosteric modulation of the dopamine receptor". European Journal of Pharmacology 641 (2–3): 96–101. September 2010. doi:10.1016/j.ejphar.2010.05.018. PMID 20639138.
- ↑ "Inhibition by L-prolyl-L-leucyl-glycinamide (PLG) of alpha-melanocyte stimulating hormone release from hypothalamic slices". Peptides 3 (6): 885–9. 1982. doi:10.1016/0196-9781(82)90055-9. PMID 6132363.
- ↑ "Inhibition by MIF-I of alpha-MSH induced increase of intraocular pressure and miosis in rabbits". Neuropeptides 12 (4): 213–7. 1988. doi:10.1016/0143-4179(88)90057-1. PMID 2907121.
- ↑ "The effect of the blockade of alpha-melanocyte-stimulating hormone on LH release in the rat". The Journal of Endocrinology 137 (2): 197–202. May 1993. doi:10.1677/joe.0.1370197. PMID 8100849.
- ↑ Sandyk R (May 1990). "MIF-induced augmentation of melatonin functions: possible relevance to mechanisms of action of MIF-1 in movement disorders". The International Journal of Neuroscience 52 (1–2): 59–65. doi:10.3109/00207459008994244. PMID 1979968.
- ↑ "MIF-1 is active in a chronic stress animal model of depression". Pharmacology Biochemistry and Behavior 32 (3): 737–42. March 1989. doi:10.1016/0091-3057(89)90027-0. PMID 2568001.
- ↑ "MIF-1 potentiates the action of tricyclic antidepressants in an animal model of depression". Peptides 12 (5): 915–8. 1991. doi:10.1016/0196-9781(91)90037-p. PMID 1686934.
- ↑ "Improvement in major depression after low subcutaneous doses of MIF-1". Journal of Affective Disorders 31 (4): 227–33. August 1994. doi:10.1016/0165-0327(94)90098-1. PMID 7989637.
- ↑ "Increased acquisition of a complex appetitive task after MSH and MIF". Pharmacology Biochemistry and Behavior 3 (5): 901–4. 1975. doi:10.1016/0091-3057(75)90124-0. PMID 1801.
- ↑ "Memory enhancement induced in chicks by L-prolyl-L-leucyl-glycinamide". Pharmacology Biochemistry and Behavior 17 (5): 893–6. November 1982. doi:10.1016/0091-3057(82)90467-1. PMID 6129646.
- ↑ "MIF-1 can accelerate neuromotor, EEG and behavioral development in mice". Peptides 11 (3): 527–32. 1990. doi:10.1016/0196-9781(90)90054-9. PMID 1974348.
- ↑ "Brain Activation by Peptide Pro-Leu-Gly-NH(2) (MIF-1)". International Journal of Peptides 2010: 1–10. 2010. doi:10.1155/2010/537639. PMID 20721355.
- ↑ "Neurological effects of MIF-1, MSH, and opiate peptides in clinical studies". International Journal of Neurology 14 (2–4): 205–9. 1980. PMID 6152908.
- ↑ "Antiparkinsonian activity of L-propyl-L-leucyl-glycinamide or melanocyte-inhibiting factor in MPTP-treated common marmosets". Movement Disorders 22 (5): 715–9. April 2007. doi:10.1002/mds.21256. PMID 17373723.
- ↑ "MIF-1 and its peptidomimetic analogs attenuate haloperidol-induced vacuous chewing movements and modulate apomorphine-induced rotational behavior in 6-hydroxydopamine-lesioned rats". Peptides 28 (10): 2009–15. October 2007. doi:10.1016/j.peptides.2007.07.026. PMID 17766011.
- ↑ "Differential metabolism of Tyr-MIF-1 and MIF-1 in rat and human plasma". Biochemical Pharmacology 47 (4): 699–709. February 1994. doi:10.1016/0006-2952(94)90133-3. PMID 7907473.
- ↑ "The Tyr-MIF-1 family of peptides". Neuroscience and Biobehavioral Reviews 18 (4): 519–25. 1994. doi:10.1016/0149-7634(94)90005-1. PMID 7708364.
- ↑ "Opposite direction of transport across the blood–brain barrier for Tyr-MIF-1 and MIF-1: comparison with morphine". Peptides 15 (1): 23–9. January 1994. doi:10.1016/0196-9781(94)90165-1. PMID 7912427.
- ↑ "Melanocyte-stimulating hormone release-inhibiting factor-1 (MIF-1) can be formed from Tyr-MIF-1 in brain mitochondria but not in brain homogenate". Journal of Neurochemistry 64 (4): 1855–9. April 1995. doi:10.1046/j.1471-4159.1995.64041855.x. PMID 7891114.
- ↑ "A potent and selective endogenous agonist for the mu-opiate receptor". Nature 386 (6624): 499–502. April 1997. doi:10.1038/386499a0. PMID 9087409. Bibcode: 1997Natur.386..499Z.
- ↑ "From MIF-1 to endomorphin: the Tyr-MIF-1 family of peptides". Peptides 28 (12): 2411–34. December 2007. doi:10.1016/j.peptides.2007.10.006. PMID 17988762.
- ↑ "Behavioral effects of neuropeptides in rodent models of depression and anxiety". Peptides 31 (4): 736–56. April 2010. doi:10.1016/j.peptides.2009.12.015. PMID 20026211.
- ↑ "PAOPA, a potent analogue of Pro-Leu-glycinamide and allosteric modulator of the dopamine D2 receptor, prevents NMDA receptor antagonist (MK-801)-induced deficits in social interaction in the rat: implications for the treatment of negative symptoms in schizophrenia". Schizophrenia Research 125 (1): 88–92. January 2011. doi:10.1016/j.schres.2010.09.025. PMID 21036015.
 |

