Chemistry:Melatonin
Melatonin, an indoleamine, is a natural compound produced by various organisms, including bacteria and eukaryotes.[1] Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs. This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates.[2][3]
In vertebrates, melatonin's functions extend to synchronizing sleep-wake cycles, encompassing sleep-wake timing and blood pressure regulation, as well as controlling seasonal rhythmicity (circannual cycle), which includes reproduction, fattening, molting, and hibernation.[4] Its effects are mediated through the activation of melatonin receptors and its role as an antioxidant.[5][6][7] In plants and bacteria, melatonin primarily serves as a defense mechanism against oxidative stress, indicating its evolutionary significance.[8] The mitochondria, key organelles within cells, are the main producers of antioxidant melatonin,[9] underscoring the molecule's "ancient origins" and its fundamental role in protecting the earliest cells from reactive oxygen species.[10][11]
In addition to its endogenous functions as a hormone and antioxidant, melatonin is also administered exogenously as a dietary supplement and medication. Melatonin is used medically primarily for sleep-related problems: for example, prolonged-release melatonin (Circadin) is approved in several countries for short-term treatment of insomnia in people over 55.[12] It is used in the treatment of sleep disorders, including insomnia and various circadian rhythm sleep disorders.
Biological activity
In humans, melatonin primarily acts as a potent full agonist of two types of melatonin receptors: melatonin receptor 1, with picomolar binding affinity, and melatonin receptor 2, with nanomolar binding affinity. Both receptors are part of the G-protein coupled receptors (GPCRs) family, specifically the Gi/o alpha subunit GPCRs,[13][14] although melatonin receptor 1 also exhibits coupling with Gq alpha subunit.[13]
Furthermore, melatonin functions as a high-capacity antioxidant, or free radical scavenger, within mitochondria, playing a dual role in combating cellular oxidative stress. First, it directly neutralizes free radicals, and second, it promotes the gene expression of essential antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase. This increase in antioxidant enzyme expression is mediated through signal transduction pathways activated by the binding of melatonin to its receptors. Through these mechanisms, melatonin protects the cell against oxidative stress in two ways, highlighting how it serves human health beyond regulating the sleep-wake cycle.[15][13][16][17][18][19]
Biological functions
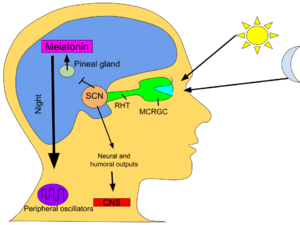
Circadian rhythm
In mammals, melatonin is critical for the regulation of sleep–wake cycles, or circadian rhythms.[20] The establishment of regular melatonin levels in human infants occurs around the third month after birth, with peak concentrations observed between midnight and 8:00 am.[21] It has been documented that melatonin production diminishes as a person ages.[22] Additionally, a shift in the timing of melatonin secretion is observed during adolescence, resulting in delayed sleep and wake times, increasing their risk for delayed sleep phase disorder during this period.[23]
The antioxidant properties of melatonin were first recognized in 1993.[24] In vitro studies reveal that melatonin directly neutralizes various reactive oxygen species, including hydroxyl (OH•), superoxide (O2−•), and reactive nitrogen species such as nitric oxide (NO•).[25][26] In plants, melatonin works synergistically with other antioxidants, enhancing the overall effectiveness of each antioxidant.[26] This compound has been found to be twice as efficacious as vitamin E, a known potent lipophilic antioxidant, at scavenging peroxyl radicals.[27] The promotion of antioxidant enzyme expression, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase, is mediated through melatonin receptor-triggered signal transduction pathways.[13][15]
Melatonin's concentration in the mitochondrial matrix is significantly higher than that found in the blood plasma,[16][17][18] emphasizing its role not only in direct free radical scavenging but also in modulating the expression of antioxidant enzymes and maintaining mitochondrial integrity. This multifaceted role shows the physiological significance of melatonin as a mitochondrial antioxidant, a notion supported by numerous scholars.[15][16][17][18][19]
Furthermore, the interaction of melatonin with reactive oxygen and nitrogen species results in the formation of metabolites capable of reducing free radicals.[13][19] These metabolites, including cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK), contribute to the broader antioxidative effects of melatonin through further redox reactions with free radicals.[13][19]
Immune system
Melatonin's interaction with the immune system is recognized, yet the specifics of these interactions remain inadequately defined.[28][29][30][31] An anti-inflammatory effect appears to be the most significant.[30][31] The efficacy of melatonin in disease treatment has been the subject of limited trials, with most available data deriving from small-scale, preliminary studies. It is posited that any beneficial immunological impact is attributable to melatonin's action on high-affinity receptors (MT1 and MT2), which are present on immunocompetent cells. Preclinical investigations suggest that melatonin may augment cytokine production and promote the expansion of T cells,[32][33] thereby potentially mitigating acquired immunodeficiencies.[34]
Weight regulation
Melatonin's potential to regulate weight gain is posited to involve its inhibitory effect on leptin, a hormone that serves as a long-term indicator of the body's energy status.[35][36] Leptin is important for regulating energy balance and body weight by signaling satiety and reducing food intake. Melatonin, by modulating leptin's actions outside of waking hours, may contribute to the restoration of leptin sensitivity during daytime, thereby counteracting leptin resistance.
Biochemistry
Biosynthesis
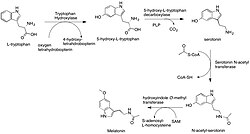
The biosynthesis of melatonin in animals involves a sequence of enzymatic reactions starting with L-tryptophan, which can be synthesized through the shikimate pathway from chorismate, found in plants, or obtained from protein catabolism. The initial step in the melatonin biosynthesis pathway is the hydroxylation of L-tryptophan's indole ring by the enzyme tryptophan hydroxylase, resulting in the formation of 5-hydroxytryptophan (5-HTP). Subsequently, 5-HTP undergoes decarboxylation, facilitated by pyridoxal phosphate and the enzyme 5-hydroxytryptophan decarboxylase, yielding serotonin.[37]
Serotonin, an essential neurotransmitter, is further converted into N-acetylserotonin by the action of serotonin N-acetyltransferase, using acetyl-CoA.[38] The final step in the pathway involves the methylation of N-acetylserotonin's hydroxyl group by hydroxyindole O-methyltransferase, with S-adenosyl methionine as the methyl donor, to produce melatonin.[38]
In bacteria, protists, fungi, and plants, the synthesis of melatonin also involves tryptophan as an intermediate but originates indirectly from the shikimate pathway. The pathway commences with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells, additionally involves carbon dioxide. While the subsequent biosynthetic reactions share similarities with those in animals, there are slight variations in the enzymes involved in the final stages.[39][40]
The hypothesis that melatonin synthesis occurs within mitochondria and chloroplasts suggests an evolutionary and functional significance of melatonin in cellular energy metabolism and defense mechanisms against oxidative stress, reflecting the molecule's ancient origins and its multifaceted roles across different domains of life.[41]
Mechanism
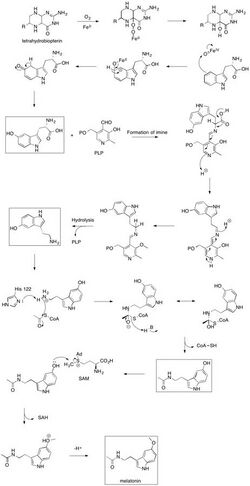
The mechanism of melatonin biosynthesis initiates with the hydroxylation of L-tryptophan, a process that requires the cofactor tetrahydrobiopterin (THB) to react with oxygen and the active site iron of tryptophan hydroxylase. Although the complete mechanism is not entirely understood, two main mechanisms have been proposed:
The first mechanism involves a slow transfer of one electron from THB to molecular oxygen (O2), potentially producing a superoxide (O−
2). This superoxide could then recombine with the THB radical to form 4a-peroxypterin. 4a-peroxypterin may either react with the active site iron (II) to create an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron, facilitating the hydroxylation of L-tryptophan.
Alternatively, the second mechanism proposes that oxygen interacts with the active site iron (II) first, forming iron (III) superoxide. This molecule could then react with THB to form an iron-peroxypterin intermediate.
Following the formation of iron (IV) oxide from the iron-peroxypterin intermediate, this oxide selectively attacks a double bond to yield a carbocation at the C5 position of the indole ring. A subsequent 1,2-shift of the hydrogen and the loss of one of the two hydrogen atoms on C5 would restore aromaticity, producing 5-hydroxy-L-tryptophan.[42]
The decarboxylation of 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine is then facilitated by a decarboxylase enzyme with pyridoxal phosphate (PLP) as a cofactor.[43] PLP forms an imine with the amino acid derivative, facilitating the breaking of the carbon–carbon bond and release of carbon dioxide. The protonation of the amine derived from tryptophan restores the aromaticity of the pyridine ring, leading to the production of 5-hydroxytryptamine and PLP.[44]
Serotonin N-acetyltransferase, with histidine residue His122, is hypothesized to deprotonate the primary amine of 5-hydroxytryptamine. This deprotonation allows the lone pair on the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The thiol from coenzyme A then acts as a leaving group when attacked by a general base, producing N-acetylserotonin.[45]
The final step in the biosynthesis of melatonin involves the methylation of N-acetylserotonin at the hydroxyl position by SAM, resulting in the production of S-adenosyl homocysteine (SAH) and melatonin.[44][46]
Regulation
In vertebrates, the secretion of melatonin is regulated through the activation of the beta-1 adrenergic receptor by the hormone norepinephrine.[47] Norepinephrine increases the concentration of intracellular cAMP via beta-adrenergic receptors, which in turn activates the cAMP-dependent protein kinase A (PKA). PKA then phosphorylates arylalkylamine N-acetyltransferase (AANAT), the penultimate enzyme in the melatonin synthesis pathway. When exposed to daylight, noradrenergic stimulation ceases, leading to the immediate degradation of the protein by proteasomal proteolysis.[48] The production of melatonin recommences in the evening, a phase known as the dim-light melatonin onset.
Blue light, especially within the 460–480 nm range, inhibits the biosynthesis of melatonin,[49] with the degree of suppression being directly proportional to the intensity and duration of light exposure. Historically, humans in temperate climates experienced limited exposure to blue daylight during winter months, primarily receiving light from sources that emitted predominantly yellow light, such as fires.[50] The incandescent light bulbs used extensively throughout the 20th century emitted relatively low levels of blue light.[51] It has been found that light containing only wavelengths greater than 530 nm does not suppress melatonin under bright-light conditions.[52] The use of glasses that block blue light in the hours preceding bedtime can mitigate melatonin suppression.[53] Additionally, wearing blue-blocking goggles during the last hours before bedtime is recommended for individuals needing to adjust to an earlier bedtime since melatonin facilitates the onset of sleep.[54]
Metabolism
Melatonin is metabolized with an elimination half-life ranging from 20 to 50 minutes.[55][2][56] The primary metabolic pathway transforms melatonin into 6-hydroxymelatonin, which is then conjugated with sulfate and excreted in urine as a waste product.[57] It is primarily metabolized by the liver enzyme CYP1A2 and to a lesser extent by CYP1A1, CYP2C19, and CYP1B1.[57]
Measurement
For both research and clinical purposes, melatonin levels in humans can be determined through saliva or blood plasma analysis.[58]
Use as a medication and supplement
Insomnia
An extended-release pharmaceutical formulation of melatonin is approved under the brand name Circadin for the treatment of insomnia in certain settings, such as in people over 55 years of age.[59][60][61][62] It is approved in the European Union, Israel, Australia, and countries in Asia and elsewhere in the world, but not in the United States (where it reached phase 3 trials but was not approved).[61][62] The medication has been licensed since 2007.[61][62]
The 2023 European Insomnia Guideline recommended use of prolonged-release melatonin for treatment of insomnia in people age 55 or older for up to 3 months.[63] It recommended against fast-release or over-the-counter melatonin for treatment of insomnia.[63] These recommendations were based on several meta-analyses published in 2022 and 2023.[63]
The American Academy of Sleep Medicine's 2017 clinical practice guidelines recommended against the use of melatonin in the treatment of insomnia due to poor effectiveness and very low quality of evidence.[64][65]
Circadian rhythm sleep disorders
Melatonin may be useful in the treatment of delayed sleep phase syndrome.[66]
Melatonin is known to reduce jet lag, especially in eastward travel. However, if it is not taken at the correct time, it can instead delay adaptation.[67]
Melatonin appears to have limited use against the sleep problems of people who work shift work.[68] Tentative evidence suggests that it increases the length of time people are able to sleep.[68]
Meta-analyses, published between 2005 and 2017, appear to show different results as to whether melatonin is effective for circadian rhythm sleep disorders or not.[69][70][71][72] Some found that it was effective,[69][70][72] while others found no evidence of effectiveness.[71] Meta-analyses of melatonin for delayed sleep phase syndrome that found it effective have reported that it improves time to sleep onset by about 40 minutes (0.67 hours) and advances onset of endogenous melatonin secretion by about 1.2 hours (72 minutes).[70][72] One meta-analysis found that melatonin was notably more effective in improving sleep onset latency in people with delayed sleep phase syndrome than in people with insomnia (improvement of 39 minutes vs. 7 minutes, respectively).[72] One meta-analysis found that melatonin was probably effective for jet lag syndrome.[73]
Low doses of melatonin may be advantageous to high doses in the treatment of sleep-cycle disorders.[74]
REM sleep behavior disorder
Melatonin is a safer alternative than clonazepam in the treatment of REM sleep behavior disorder – a condition associated with the synucleinopathies like Parkinson's disease and dementia with Lewy bodies.[75][76][77] However, clonazepam may be more effective.[78] In any case, the quality of evidence for both treatments is very low and it is unclear whether either is definitely effective.[78]
Dementia
A 2020 Cochrane review found no evidence that melatonin helped sleep problems in people with moderate to severe dementia due to Alzheimer's disease.[79] A 2019 review found that while melatonin may improve sleep in minimal cognitive impairment, after the onset of Alzheimer's disease it has little to no effect.[80] Melatonin may, however, help with sundowning (increased confusion and restlessness at night) in people with dementia.[81]
Available forms

A prolonged-release 2 mg oral formulation of melatonin sold under the brand name Circadin is approved for use in the European Union in the short-term treatment of insomnia in people age 55 and older.[59][60][82]
Melatonin is also available as an over-the-counter dietary supplement in many countries. It is available in both immediate-release and less commonly prolonged-release forms. The compound is available in supplements at doses ranging from 0.3 mg to 10 mg or more. It is also possible to buy raw melatonin powder by weight.[83] Immediate-release formulations of melatonin cause blood levels of melatonin to reach their peak in about an hour. The hormone may be administered orally, as capsules, gummies, tablets, oral films, or as a liquid.[84] It is also available for use sublingually, or as transdermal patches.[85] Several inhalation-based melatonin products with a wide range of doses are available but their safety remains to be evaluated.[84]
The American Academy of Sleep Medicine (AASM) says that the melatonin content in unregulated (without a USP verified mark) supplements can diverge widely from the claimed amount; a study found that the melatonin content ranged from one half to four times the stated dose.[86]
History
Discovery
Melatonin's discovery is linked to the study of skin color changes in some amphibians and reptiles, a phenomenon initially observed through the administration of pineal gland extracts.[87][88] In 1917, Carey Pratt McCord and Floyd P. Allen found that feeding extracts from the pineal glands of cows caused the skin of tadpoles to lighten by contracting the dark epidermal melanophores.[89][90]
The hormone melatonin was isolated in 1958 by Aaron B. Lerner, a dermatology professor, and his team at Yale University. Motivated by the possibility that a substance from the pineal gland could be beneficial in treating skin diseases, they extracted and identified melatonin from bovine pineal gland extracts.[91] Subsequent research in the mid-1970s by Lynch and others demonstrated that melatonin production follows a circadian rhythm in human pineal glands.[92]
The first patent for the therapeutic use of melatonin as a low-dose sleep aid was awarded to Richard Wurtman at the Massachusetts Institute of Technology in 1995.[93]
Etymology
The etymology of melatonin stems from its skin-lightening properties. As detailed in their publication in the Journal of the American Chemical Society,[94] Lerner and his colleagues proposed the name melatonin, derived from the Greek words melas, meaning 'black' or 'dark', and tonos, meaning 'labour',[95] 'colour'[96] or 'suppress'.[97] This naming convention follows that of serotonin, another agent affecting skin color, discovered in 1948 as a modulator of vascular tone, which influenced its name based on its serum vasoconstrictor effect.[98] Melatonin was thus aptly named to reflect its role in preventing the darkening of the skin, highlighting the intersection of biochemistry and linguistics in scientific discovery.[94]
Occurrence
Animals and Humans
In vertebrates, melatonin is produced in darkness, thus usually at night, by the pineal gland, a small endocrine gland[99] located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the suprachiasmatic nuclei from retinal photosensitive ganglion cells of the eyes[100][101] rather than the melatonin signal (as was once postulated). Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.[102]
In humans, ~30 μg of melatonin is produced daily and 80% of the total amount is produced in the night (W). The plasma maximum concentration of melatonin at night are 80–120 pg/mL and the concentrations during the day are between 10–20 pg/mL.[103][104]
Many animals and humans use the variation in duration of melatonin production each day as a seasonal clock.[105] In animals including humans,[106] the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage coloring in seasonal animals.[106] In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including mynah birds[107] and hamsters.[108] Melatonin can suppress libido by inhibiting secretion of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is stimulated by melatonin. In sheep, melatonin administration has also shown antioxidant and immune-modulatory regime in prenatally stressed offspring helping them survive the crucial first days of their lives.[109]
During the night, melatonin regulates leptin, lowering its levels.
Cetaceans have lost all the genes for melatonin synthesis as well as those for melatonin receptors.[110] This is thought to be related to their unihemispheric sleep pattern (one brain hemisphere at a time). Similar trends have been found in sirenians.[110]
Plants
Until its identification in plants in 1987, melatonin was for decades thought to be primarily an animal neurohormone. When melatonin was identified in coffee extracts in the 1970s, it was believed to be a byproduct of the extraction process. Subsequently, however, melatonin has been found in all plants that have been investigated. It is present in all the different parts of plants, including leaves, stems, roots, fruits, and seeds, in varying proportions.[8][111] Melatonin concentrations differ not only among plant species, but also between varieties of the same species depending on the agronomic growing conditions, varying from picograms to several micrograms per gram.[40][112] Notably high melatonin concentrations have been measured in popular beverages such as coffee, tea, wine, and beer, and crops including corn, rice, wheat, barley, and oats.[8] In some common foods and beverages, including coffee[8] and walnuts,[113] the concentration of melatonin has been estimated or measured to be sufficiently high to raise the blood level of melatonin above daytime baseline values.
Although a role for melatonin as a plant hormone has not been clearly established, its involvement in processes such as growth and photosynthesis is well established. Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described. Rather, melatonin performs important roles in plants as a growth regulator, as well as environmental stress protector. It is synthesized in plants when they are exposed to both biological stresses, for example, fungal infection, and nonbiological stresses such as extremes of temperature, toxins, increased soil salinity, drought, etc.[40][114][115]
Herbicide-induced oxidative stress has been experimentally mitigated in vivo in a high-melatonin transgenic rice.[116][117][118] Studies conducted on lettuce grown in saline soil conditions have shown that the application of melatonin significantly mitigates the harmful effects of salinity. Foliar application increases the number of leaves, their surface area, increases fresh weight and the content of chlorophyll a and chlorophyll b, and the content of carotenoids compared to plants not treated with melatonin.[118]
Fungal disease resistance is another role. Added melatonin increases resistance in Malus prunifolia against Diplocarpon mali.[117][119] Also acts as a growth inhibitor on fungal pathogens including Alternaria, Botrytis, and Fusarium spp. Decreases the speed of infection. As a seed treatment, protects Lupinus albus from fungi. Dramatically slows Pseudomonas syringae tomato DC3000 infecting Arabidopsis thaliana and infecting Nicotiana benthamiana.[119]
Fungi
Melatonin has been observed to reduce stress tolerance in Phytophthora infestans in plant-pathogen systems.[120] Danish pharmaceutical company Novo Nordisk have used genetically modified yeast (Saccharomyces cerevisiae) to produce melatonin.[121]
Bacteria
Melatonin is produced by α-proteobacteria and photosynthetic cyanobacteria. There is no report of its occurrence in archaea which indicates that melatonin originated in bacteria[11] most likely to prevent the first cells from the damaging effects of oxygen in the primitive Earth's atmosphere.[10]
Novo Nordisk have used genetically modified Escherichia coli to produce melatonin.[122][123]
Archaea
In 2022, the discovery of serotonin N-acetyltransferase (SNAT)—the penultimate, rate-limiting enzyme in the melatonin biosynthetic pathway—in the archaeon Thermoplasma volcanium[124] firmly places melatonin biosynthesis in all three major domains of life, dating back to ~4 Gya.[125]
Food products
Naturally occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g,[126] bananas, plums, grapes, rice, cereals, herbs,[127] olive oil, wine,[128] and beer.[129] The consumption of milk and sour cherries may improve sleep quality.[130] When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains.[131] When humans consume foods rich in melatonin, such as banana, pineapple, and orange, the blood levels of melatonin increase significantly.[132]
References
- ↑ Amaral, Fernanda Gaspar do; Cipolla-Neto, José (2018). "A brief review about melatonin, a pineal hormone". Archives of Endocrinology and Metabolism 62 (4): 472–479. doi:10.20945/2359-3997000000066. PMID 30304113.
- ↑ 2.0 2.1 "Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders". Sleep Medicine Reviews 34: 10–22. August 2017. doi:10.1016/j.smrv.2016.06.005. PMID 28648359. http://epubs.surrey.ac.uk/813219/1/Riha%20accepted%20MS%202016.pdf.
- ↑ ADHD: Non-Pharmacologic Interventions, An Issue of Child and Adolescent Psychiatric Clinics of North America, E-Book. Elsevier Health Sciences. 2014. p. 888. ISBN 978-0-323-32602-5. https://books.google.com/books?id=lNSlBAAAQBAJ&pg=PA888.
- ↑ "Melatonin: therapeutic and clinical utilization". International Journal of Clinical Practice 61 (5): 835–45. May 2007. doi:10.1111/j.1742-1241.2006.01191.x. PMID 17298593.
- ↑ "Molecular tools to study melatonin pathways and actions". Trends in Pharmacological Sciences 26 (8): 412–9. August 2005. doi:10.1016/j.tips.2005.06.006. PMID 15992934.
- ↑ "Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance". Endocrine 27 (2): 119–30. July 2005. doi:10.1385/ENDO:27:2:119. PMID 16217125.
- ↑ "Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system". Annals of the New York Academy of Sciences 939 (1): 200–15. June 2001. doi:10.1111/j.1749-6632.2001.tb03627.x. PMID 11462772. Bibcode: 2001NYASA.939..200R.
- ↑ 8.0 8.1 8.2 8.3 "Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science". Journal of Experimental Botany 63 (2): 577–97. January 2012. doi:10.1093/jxb/err256. PMID 22016420.
- ↑ Reiter, Russel J.; Tan, Dun Xian; Rosales-Corral, Sergio; Galano, Annia; Zhou, Xin Jia; Xu, Bing (2018). "Mitochondria: Central Organelles for Melatonin's Antioxidant and Anti-Aging Actions". Molecules 23 (2): 509. doi:10.3390/molecules23020509. PMID 29495303.
- ↑ 10.0 10.1 Manchester, Lucien C.; Coto-Montes, Ana; Boga, Jose Antonio; Andersen, Lars Peter H.; Zhou, Zhou; Galano, Annia; Vriend, Jerry; Tan, Dun-Xian et al. (2015). "Melatonin: an ancient molecule that makes oxygen metabolically tolerable". Journal of Pineal Research 59 (4): 403–419. doi:10.1111/jpi.12267. PMID 26272235.
- ↑ 11.0 11.1 Zhao, Dake; Yu, Yang; Shen, Yong; Liu, Qin; Zhao, Zhiwei; Sharma, Ramaswamy; Reiter, Russel J. (2019). "Melatonin Synthesis and Function: Evolutionary History in Animals and Plants". Frontiers in Endocrinology 10. doi:10.3389/fendo.2019.00249. PMID 31057485.
- ↑ "The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023". J Sleep Res 32 (6). December 2023. doi:10.1111/jsr.14035. PMID 38016484.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 "Update on melatonin receptors: IUPHAR Review 20". British Journal of Pharmacology 173 (18): 2702–25. September 2016. doi:10.1111/bph.13536. PMID 27314810. "Hence, one melatonin molecule and its associated metabolites could scavenge a large number of reactive species, and thus, the overall antioxidant capacity of melatonin is believed to be greater than that of other well-known antioxidants, such as vitamin C and vitamin E, under in vitro or in vivo conditions".
- ↑ "Melatonin receptors | G protein-coupled receptors | IUPHAR/BPS Guide to Pharmacology". http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39.
- ↑ 15.0 15.1 15.2 "Melatonin and human mitochondrial diseases". Journal of Research in Medical Sciences 22: 2. 2017. doi:10.4103/1735-1995.199092. PMID 28400824.
- ↑ 16.0 16.1 16.2 "Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas". Cellular and Molecular Life Sciences 74 (21): 3863–3881. November 2017. doi:10.1007/s00018-017-2609-7. PMID 28864909. "melatonin is specifically targeted to the mitochondria where it seems to function as an apex antioxidant ... The measurement of the subcellular distribution of melatonin has shown that the concentration of this indole in the mitochondria greatly exceeds that in the blood.".
- ↑ 17.0 17.1 17.2 "Melatonin as an antioxidant: under promises but over delivers". Journal of Pineal Research 61 (3): 253–78. October 2016. doi:10.1111/jpi.12360. PMID 27500468.
- ↑ 18.0 18.1 18.2 "Melatonin: an ancient molecule that makes oxygen metabolically tolerable". Journal of Pineal Research 59 (4): 403–19. November 2015. doi:10.1111/jpi.12267. PMID 26272235. "While originally thought to be produced exclusively in and secreted from the vertebrate pineal gland, it is now known that the indole is present in many, perhaps all, vertebrate organs".
- ↑ 19.0 19.1 19.2 19.3 "Melatonin transport into mitochondria". Cellular and Molecular Life Sciences 74 (21): 3927–3940. November 2017. doi:10.1007/s00018-017-2616-8. PMID 28828619.
- ↑ "A Review of Melatonin, Its Receptors and Drugs". The Eurasian Journal of Medicine 48 (2): 135–41. June 2016. doi:10.5152/eurasianjmed.2015.0267. PMID 27551178.
- ↑ "Emergence and evolution of the circadian rhythm of melatonin in children". Hormone Research 59 (2): 66–72. 2003. doi:10.1159/000068571. PMID 12589109.
- ↑ "Human melatonin production decreases with age". Journal of Pineal Research 3 (4): 379–88. 1986. doi:10.1111/j.1600-079X.1986.tb00760.x. PMID 3783419.
- ↑ "Adolescent changes in the homeostatic and circadian regulation of sleep". Developmental Neuroscience 31 (4): 276–84. June 2009. doi:10.1159/000216538. PMID 19546564.
- ↑ "Melatonin: a potent, endogenous hydroxyl radical scavenger.". Endocr. J. 1: 57–60. 1993. https://docs.google.com/document/d/e/2PACX-1vSHolKyTREzsC-RB0H-brwbUhaVP4EZBRSoZ6F7b4cOcAkutpNX3ebh0yd_QKEWRBTYVLcqpmMit3NL/pub.
- ↑ "Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro". Annals of the New York Academy of Sciences 738 (1): 419–20. November 1994. doi:10.1111/j.1749-6632.1994.tb21831.x. PMID 7832450. Bibcode: 1994NYASA.738..419P.
- ↑ 26.0 26.1 "The physiological function of melatonin in plants". Plant Signaling & Behavior 1 (3): 89–95. May 2006. doi:10.4161/psb.1.3.2640. PMID 19521488. Bibcode: 2006PlSiB...1...89A.
- ↑ "Melatonin: a peroxyl radical scavenger more effective than vitamin E". Life Sciences 55 (15): PL271-6. 1994. doi:10.1016/0024-3205(94)00666-0. PMID 7934611.
- ↑ "A review of the multiple actions of melatonin on the immune system". Endocrine 27 (2): 189–200. July 2005. doi:10.1385/ENDO:27:2:189. PMID 16217132.
- ↑ "[Immunotropic properties of pineal melatonin]" (in ru). Eksperimental'naia i Klinicheskaia Farmakologiia 65 (5): 73–80. 2002. PMID 12596522.
- ↑ 30.0 30.1 "Melatonin: buffering the immune system". Int J Mol Sci 14 (4): 8638–83. April 2013. doi:10.3390/ijms14048638. PMID 23609496.
- ↑ 31.0 31.1 "Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials". Brain Behav Immun 93: 245–253. March 2021. doi:10.1016/j.bbi.2021.01.034. PMID 33581247.
- ↑ "The modulatory role of melatonin on immune responsiveness". Current Opinion in Investigational Drugs 7 (5): 423–31. May 2006. PMID 16729718.
- ↑ "Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials". Eur J Nutr 59 (5): 1803–1813. August 2020. doi:10.1007/s00394-019-02123-0. PMID 31679041.
- ↑ "The immunotherapeutic potential of melatonin". Expert Opinion on Investigational Drugs 10 (3): 467–76. March 2001. doi:10.1517/13543784.10.3.467. PMID 11227046.
- ↑ "Protective Effects of Melatonin against Obesity-Induced by Leptin Resistance". Behavioural Brain Research 417. January 2022. doi:10.1016/j.bbr.2021.113598. PMID 34563600.
- ↑ "Narrative review: the role of leptin in human physiology: emerging clinical applications". Annals of Internal Medicine 152 (2): 93–100. January 2010. doi:10.7326/0003-4819-152-2-201001190-00008. PMID 20083828.
- ↑ "MetaCyc serotonin and melatonin biosynthesis". http://www.metacyc.org/META/new-image?type=PATHWAY&object=PWY-6030&detail-level=2&ENZORG=TAX-9606.
- ↑ 38.0 38.1 "Melatonin: Pharmacology, Functions and Therapeutic Benefits". Current Neuropharmacology 15 (3): 434–443. April 2017. doi:10.2174/1570159X14666161228122115. PMID 28503116.
- ↑ "Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources". Journal of Chemical Biology 5 (1): 5–17. January 2012. doi:10.1007/s12154-011-0064-8. PMID 22826715.
- ↑ 40.0 40.1 40.2 "Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions". Journal of Experimental Botany 66 (3): 627–46. February 2015. doi:10.1093/jxb/eru386. PMID 25240067.
- ↑ "Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes". Journal of Pineal Research 54 (2): 127–38. March 2013. doi:10.1111/jpi.12026. PMID 23137057.
- ↑ "Mechanisms of tryptophan and tyrosine hydroxylase". IUBMB Life 65 (4): 350–7. April 2013. doi:10.1002/iub.1144. PMID 23441081.
- ↑ "Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis". Biochemistry 31 (8): 2229–38. March 1992. doi:10.1021/bi00123a004. PMID 1540578.
- ↑ 44.0 44.1 Medicinal Natural Products. A Biosynthetic Approach (2nd ed.). Wiley. 2002. ISBN 978-0-471-49640-3.
- ↑ "Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism". Molecular Cell 3 (1): 23–32. January 1999. doi:10.1016/S1097-2765(00)80171-9. PMID 10024876.
- ↑ "Human hydroxyindole-O-methyltransferase: presence of LINE-1 fragment in a cDNA clone and pineal mRNA". DNA and Cell Biology 12 (8): 715–27. October 1993. doi:10.1089/dna.1993.12.715. PMID 8397829. https://zenodo.org/record/1235255.
- ↑ "Headache, drugs and sleep". Cephalalgia 34 (10): 756–66. September 2014. doi:10.1177/0333102414542662. PMID 25053748.
- ↑ "Mechanisms regulating melatonin synthesis in the mammalian pineal organ". Annals of the New York Academy of Sciences 1057 (1): 372–83. December 2005. doi:10.1196/annals.1356.028. PMID 16399907. Bibcode: 2005NYASA1057..372S.
- ↑ "Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor". The Journal of Neuroscience 21 (16): 6405–12. August 2001. doi:10.1523/JNEUROSCI.21-16-06405.2001. PMID 11487664.
- ↑ "What's in a color? The unique human health effect of blue light". Environmental Health Perspectives 118 (1): A22-7. January 2010. doi:10.1289/ehp.118-a22. PMID 20061218.
- ↑ "Recent News – Program of Computer Graphics". https://www.graphics.cornell.edu/online/measurements/source-spectra/index.html.
- ↑ "Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work". The Journal of Clinical Endocrinology and Metabolism 90 (5): 2755–61. May 2005. doi:10.1210/jc.2004-2062. PMID 15713707.
- ↑ "University of Houston study shows blue light glasses at night increase melatonin by 58%" (in en). 25 August 2021. https://designeroptics.com/blogs/news/university-of-houston-study-shows-blue-light-glasses-at-night-increase-melatonin-by-58.
- ↑ "Amber lenses to block blue light and improve sleep: a randomized trial". Chronobiology International 26 (8): 1602–12. December 2009. doi:10.3109/07420520903523719. PMID 20030543.
- ↑ "Melatonin". https://www.drugbank.ca/drugs/DB01065.
- ↑ "Melatonergic drugs in clinical practice". Arzneimittelforschung 58 (1): 1–10. 2008. doi:10.1055/s-0031-1296459. PMID 18368944.
- ↑ 57.0 57.1 Ma, Xiaochao; Idle, Jeffrey R.; Krausz, Kristopher W.; Gonzalez, Frank J. (April 2005). "Metabolism of Melatonin by Human Cytochromes P450". Drug Metabolism and Disposition 33 (4): 489–494. doi:10.1124/dmd.104.002410. PMID 15616152. https://dmd.aspetjournals.org/content/33/4/489. Retrieved 25 January 2023.
- ↑ "A critical review of melatonin assays: Past and present". Journal of Pineal Research 67 (1). August 2019. doi:10.1111/jpi.12572. PMID 30919486.
- ↑ 59.0 59.1 "Melatonin prolonged release: in the treatment of insomnia in patients aged ≥55 years". Drugs & Aging 29 (11): 911–23. November 2012. doi:10.1007/s40266-012-0018-z. PMID 23044640.
- ↑ 60.0 60.1 "Prolonged-release melatonin for the treatment of insomnia in patients over 55 years". Expert Opin Investig Drugs 17 (10): 1567–72. October 2008. doi:10.1517/13543784.17.10.1567. PMID 18808316.
- ↑ 61.0 61.1 61.2 "Circadin". 9 October 2015. https://www.alzforum.org/therapeutics/circadin.
- ↑ 62.0 62.1 62.2 "Melatonin controlled-release". 8 April 2025. https://adisinsight.springer.com/drugs/800018177.
- ↑ 63.0 63.1 63.2 "The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023". J Sleep Res 32 (6). December 2023. doi:10.1111/jsr.14035. PMID 38016484.
- ↑ "Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline". J Clin Sleep Med 13 (2): 307–349. February 2017. doi:10.5664/jcsm.6470. PMID 27998379.
- ↑ "Chapter 10: Insomnia". Pseudoscience in Therapy: A Skeptical Field Guide. Cambridge University Press. 2023. pp. 147–148. doi:10.1017/9781009000611.011. ISBN 978-1-009-00061-1.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAuld20172 - ↑ "Melatonin for the prevention and treatment of jet lag". The Cochrane Database of Systematic Reviews 2010 (2). 2002. doi:10.1002/14651858.CD001520. PMID 12076414.
- ↑ 68.0 68.1 "Pharmacological interventions for sleepiness and sleep disturbances caused by shift work". The Cochrane Database of Systematic Reviews 2014 (8). August 2014. doi:10.1002/14651858.CD009776.pub2. PMID 25113164.
- ↑ 69.0 69.1 "Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders". Sleep Med Rev 34: 10–22. August 2017. doi:10.1016/j.smrv.2016.06.005. PMID 28648359.
- ↑ 70.0 70.1 70.2 "The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis". Sleep 33 (12): 1605–14. December 2010. doi:10.1093/sleep/33.12.1605. PMID 21120122.
- ↑ 71.0 71.1 "Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis". BMJ 332 (7538): 385–393. February 2006. doi:10.1136/bmj.38731.532766.F6. PMID 16473858.
- ↑ 72.0 72.1 72.2 72.3 "The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis". J Gen Intern Med 20 (12): 1151–1158. December 2005. doi:10.1111/j.1525-1497.2005.0243.x. PMID 16423108.
- ↑ "Is melatonin useful for jet lag?". Medwave 15 Suppl 3. December 2015. doi:10.5867/medwave.2015.6343. PMID 26731279.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedZawilskaSkeneArendt2009 - ↑ "Treatment outcomes in REM sleep behavior disorder". Sleep Medicine 14 (3): 237–42. March 2013. doi:10.1016/j.sleep.2012.09.018. PMID 23352028.
- ↑ "Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium". Neurology 89 (1): 88–100. July 2017. doi:10.1212/WNL.0000000000004058. PMID 28592453.
- ↑ "Comprehensive treatment of dementia with Lewy bodies". Alzheimer's Research & Therapy 7 (1). 2015. doi:10.1186/s13195-015-0128-z. PMID 26029267.
- ↑ 78.0 78.1 "A critical review of the pharmacological treatment of REM sleep behavior disorder in adults: time for more and larger randomized placebo-controlled trials". J Neurol 269 (1): 125–148. January 2022. doi:10.1007/s00415-020-10353-0. PMID 33410930.
- ↑ "Pharmacotherapies for sleep disturbances in dementia". The Cochrane Database of Systematic Reviews 2020 (11). November 2020. doi:10.1002/14651858.CD009178.pub4. PMID 33189083.
- ↑ "Neuroendocrine-Metabolic Dysfunction and Sleep Disturbances in Neurodegenerative Disorders: Focus on Alzheimer's Disease and Melatonin". Neuroendocrinology 108 (4): 354–364. 2019. doi:10.1159/000494889. PMID 30368508.
- ↑ "Mild Cognitive Impairment and Dementia". Handbook of Sleep Disorders in Medical Conditions. Academic Press. 2019. pp. 253–276. doi:10.1016/b978-0-12-813014-8.00011-1. ISBN 978-0-12-813014-8.
- ↑ Circadin: EPAR - Product Information ANNEX I - SUMMARY OF PRODUCT CHARACTERISTICS (Report). European Medicines Agency (EMA). 2 February 2021. EMEA/H/C/000695 - IA/0066. https://www.ema.europa.eu/en/medicines/human/EPAR/circadin. As PDF. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Melatonin Product Availability". 28 February 2021. https://keldik.com/blogs/sleep-circadian-binnacle/melatonin-product-availability.
- ↑ 84.0 84.1 "Simulated pharmacokinetics of inhaled caffeine and melatonin from existing products indicate the lack of dosimetric considerations". Food and Chemical Toxicology 187. May 2024. doi:10.1016/j.fct.2024.114601. PMID 38493979.
- ↑ "Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action". BMC Medicine 16 (1). February 2018. doi:10.1186/s12916-017-1000-8. PMID 29397794.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedbrooks - ↑ "Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates". Journal of Pineal Research 20 (4): 175–86. May 1996. doi:10.1111/j.1600-079X.1996.tb00256.x. PMID 8836950.
- ↑ "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Research 17 (5): 454–60. October 2004. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- ↑ Encyclopedia of dietary supplements. New York, N.Y: Marcel Dekker. 2005. pp. 457–66. ISBN 978-0-8247-5504-1. https://books.google.com/books?id=Sfmc-fRCj10C&q=Lerner+melatonin+history&pg=PA457.
- ↑ "Evidences associating pineal gland function with alterations in pigmentation". J Exp Zool 23 (1): 206–24. January 1917. doi:10.1002/jez.1400230108. Bibcode: 1917JEZ....23..207M. https://books.google.com/books?id=OOM1AQAAMAAJ&pg=PA207.
- ↑ "Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands". The Journal of Biological Chemistry 235 (7): 1992–7. July 1960. doi:10.1016/S0021-9258(18)69351-2. PMID 14415935.
- ↑ "Daily rhythm in human urinary melatonin". Science 187 (4172): 169–71. January 1975. doi:10.1126/science.1167425. PMID 1167425. Bibcode: 1975Sci...187..169L.
- ↑ Wurtman RJ, "Methods of inducing sleep using melatonin", US patent 5449683, issued 12 September 1995, assigned to Massachusetts Institute of Technology
- ↑ 94.0 94.1 Lerner, Aaron B.; Case, James D.; Takahashi, Yoshiyata; Lee, Teh H.; Mori, Wataru (1958). "Isolation of melatonin, the pineal gland factor that lightens melanocytes" (in en). Journal of the American Chemical Society 80 (10): 2587. doi:10.1021/ja01543a060. ISSN 0002-7863. Bibcode: 1958JAChS..80Q2587L. https://pubs.acs.org/doi/abs/10.1021/ja01543a060.
- ↑ Goeser, Suzanne; Ruble, James; Chandler, Linda (1997). "Melatonin: Historical and Clinical Perspectives" (in en). Journal of Pharmaceutical Care in Pain & Symptom Control 5 (1): 37–49. doi:10.1300/J088v05n01_04. http://www.tandfonline.com/doi/full/10.1300/J088v05n01_04.
- ↑ Beyer, C. E.; Steketee, J. D.; Saphier, D. (1998). "Antioxidant properties of melatonin–an emerging mystery". Biochemical Pharmacology 56 (10): 1265–1272. doi:10.1016/s0006-2952(98)00180-4. ISSN 0006-2952. PMID 9825724.
- ↑ Liebmann, P. M.; Wölfler, A.; Felsner, P.; Hofer, D.; Schauenstein, K. (1997). "Melatonin and the immune system". International Archives of Allergy and Immunology 112 (3): 203–211. doi:10.1159/000237455. ISSN 1018-2438. PMID 9066504.
- ↑ "Serum vasoconstrictor, serotonin; isolation and characterization". The Journal of Biological Chemistry 176 (3): 1243–1251. December 1948. doi:10.1016/S0021-9258(18)57137-4. PMID 18100415.
- ↑ "Pineal melatonin: cell biology of its synthesis and of its physiological interactions". Endocrine Reviews 12 (2): 151–80. May 1991. doi:10.1210/edrv-12-2-151. PMID 1649044.
- ↑ "The human circadian system in normal and disordered sleep". The Journal of Clinical Psychiatry 66 (Suppl 9): 3–9; quiz 42–3. 2005. PMID 16336035.
- ↑ "The biological clock: the bodyguard of temporal homeostasis". Chronobiology International 21 (1): 1–25. January 2004. doi:10.1081/CBI-120027984. PMID 15129821.
- ↑ "Sleep, circadian rhythms and health". Interface Focus 10 (3). June 2020. doi:10.1098/rsfs.2019.0098. PMID 32382406.
- ↑ Karasek, M.; Winczyk, K. (2006). "Melatonin in humans". Journal of Physiology and Pharmacology 57 Suppl 5: 19–39. ISSN 1899-1505. PMID 17218758.
- ↑ Kolli, Aditya R.; Kuczaj, Arkadiusz K.; Calvino-Martin, Florian; Hoeng, Julia (2024). "Simulated pharmacokinetics of inhaled caffeine and melatonin from existing products indicate the lack of dosimetric considerations". Food and Chemical Toxicology 187. doi:10.1016/j.fct.2024.114601. ISSN 0278-6915. PMID 38493979.
- ↑ "Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis". The Journal of Endocrinology 179 (1): 1–13. October 2003. doi:10.1677/joe.0.1790001. PMID 14529560.
- ↑ 106.0 106.1 "Melatonin as a chronobiotic". Sleep Medicine Reviews 9 (1): 25–39. February 2005. doi:10.1016/j.smrv.2004.05.002. PMID 15649736. "Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn), it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times.".
- ↑ "Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis". Australian Journal of Zoology 32 (6): 803–09. 1984. doi:10.1071/ZO9840803.
- ↑ "Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to melatonin inhibition". Neuroendocrinology 33 (1): 43–6. July 1981. doi:10.1159/000123198. PMID 7254478.
- ↑ Bouroutzika, Efterpi; Ciliberti, Maria Giovanna; Caroprese, Mariangela; Theodosiadou, Ekaterini; Papadopoulos, Serafeim; Makri, Sotiria; Skaperda, Zoi-Vasiliki; Kotsadam, Georgios et al. (2021-11-05). "Association of Melatonin Administration in Pregnant Ewes with Growth, Redox Status and Immunity of Their Offspring" (in en). Animals 11 (11): 3161. doi:10.3390/ani11113161. ISSN 2076-2615. PMID 34827893.
- ↑ 110.0 110.1 "Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations". Science Advances 5 (9). September 2019. doi:10.1126/sciadv.aaw6671. PMID 31579821. Bibcode: 2019SciA....5.6671H.
- ↑ "Phytomelatonin: a review". Journal of Experimental Botany 60 (1): 57–69. 1 January 2009. doi:10.1093/jxb/ern284. PMID 19033551.
- ↑ "Melatonin: action as antioxidant and potential applications in human disease and aging". Toxicology 278 (1): 55–67. November 2010. doi:10.1016/j.tox.2010.04.008. PMID 20417677. Bibcode: 2010Toxgy.278...55B.
- ↑ "Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood". Nutrition 21 (9): 920–4. September 2005. doi:10.1016/j.nut.2005.02.005. PMID 15979282.
- ↑ "Phytomelatonin: assisting plants to survive and thrive". Molecules 20 (4): 7396–437. April 2015. doi:10.3390/molecules20047396. PMID 25911967.
- ↑ "Functions of melatonin in plants: a review". Journal of Pineal Research 59 (2): 133–50. September 2015. doi:10.1111/jpi.12253. PMID 26094813.
- ↑ "Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress". Journal of Pineal Research (Wiley) 54 (3): 258–63. April 2013. doi:10.1111/j.1600-079x.2012.01029.x. PMID 22856683.
- ↑ 117.0 117.1 "Melatonin: plant growth regulator and/or biostimulator during stress?". Trends in Plant Science (Elsevier) 19 (12): 789–97. December 2014. doi:10.1016/j.tplants.2014.07.006. PMID 25156541. Bibcode: 2014TPS....19..789A.
- ↑ 118.0 118.1 EL-Bauome, Hemat A.; Doklega, Samar M.; Saleh, Said A.; Mohamed, Ahmed S.; Suliman, Ahmad A.; Abd El-Hady, Mahmoud A.M. (February 2024). "Effects of melatonin on lettuce plant growth, antioxidant enzymes and photosynthetic pigments under salinity stress conditions". Folia Horticulturae (Polish Society of Horticultural Science) 36 (1): 1–17. doi:10.2478/fhort-2024-0001.
- ↑ 119.0 119.1 "Functions of melatonin in plants: a review". Journal of Pineal Research (Wiley) 59 (2): 133–50. September 2015. doi:10.1111/jpi.12253. PMID 26094813.
- ↑ "Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases". Reviews in Endocrine & Metabolic Disorders 21 (4): 465–478. December 2020. doi:10.1007/s11154-020-09570-9. PMID 32691289.
- ↑ Germann, Susanne M.; Baallal Jacobsen, Simo A.; Schneider, Konstantin; Harrison, Scott J.; Jensen, Niels B.; Chen, Xiao; Stahlhut, Steen G.; Borodina, Irina et al. (2016). "Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae" (in en). Biotechnology Journal 11 (5): 717–724. doi:10.1002/biot.201500143. PMID 26710256.
- ↑ Luo, Hao; Schneider, Konstantin; Christensen, Ulla; Lei, Yang; Herrgard, Markus; Palsson, Bernhard Ø. (2020). "Microbial Synthesis of Human-Hormone Melatonin at Gram Scales" (in en). ACS Synthetic Biology 9 (6): 1240–1245. doi:10.1021/acssynbio.0c00065. ISSN 2161-5063. PMID 32501000. https://pubs.acs.org/doi/10.1021/acssynbio.0c00065.
- ↑ Arnao, Marino B.; Giraldo-Acosta, Manuela; Castejón-Castillejo, Ana; Losada-Lorán, Marta; Sánchez-Herrerías, Pablo; El Mihyaoui, Amina; Cano, Antonio; Hernández-Ruiz, Josefa (2023). "Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin". Metabolites 13 (1): 72. doi:10.3390/metabo13010072. PMID 36676997.
- ↑ Lee, Kyungjin; Choi, Geun-Hee; Back, Kyoungwhan (2022-03-21). "Functional Characterization of Serotonin N-Acetyltransferase in Archaeon Thermoplasma volcanium". Antioxidants 11 (3): 596. doi:10.3390/antiox11030596. ISSN 2076-3921. PMID 35326246.
- ↑ Hoshino, Yosuke; Villanueva, Laura (2023-03-10). "Four billion years of microbial terpenome evolution". FEMS Microbiology Reviews 47 (2). doi:10.1093/femsre/fuad008. ISSN 1574-6976. PMID 36941124.
- ↑ "Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus)". Journal of Agricultural and Food Chemistry 49 (10): 4898–902. October 2001. doi:10.1021/jf010321. PMID 11600041.
- ↑ "Ingestion of Japanese plums (Prunus salicina Lindl. cv. Crimson Globe) increases the urinary 6-sulfatoxymelatonin and total antioxidant capacity levels in young, middle-aged and elderly humans: Nutritional and functional characterization of their content". Journal of Food and Nutrition Research 50 (4): 229–36. 2011. https://www.researchgate.net/publication/259983119.
- ↑ "Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection". Journal of Pineal Research 50 (4): 374–80. May 2011. doi:10.1111/j.1600-079X.2010.00853.x. PMID 21342247.
- ↑ "Melatonin in Medicinal and Food Plants". Cells 681. 5 July 2019. https://schlaf.fit/Melatonin_in_Plants_and_Food.pdf. Retrieved 2 July 2021.
- ↑ "Influence of Dietary Sources of Melatonin on Sleep Quality: A Review". Journal of Food Science (Wiley) 85 (1): 5–13. January 2020. doi:10.1111/1750-3841.14952. PMID 31856339.
- ↑ "Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates". Biochemistry and Molecular Biology International 35 (3): 627–34. March 1995. PMID 7773197.
- ↑ "Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers". Journal of Pineal Research 55 (1): 58–64. August 2013. doi:10.1111/jpi.12025. PMID 23137025.
External links
 |
