Chemistry:4-Nitrobenzaldehyde
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Nitrobenzaldehyde | |||
| Other names
p-Nitrobenzaldehyde
| |||
| Properties | |||
| C7H5NO3 | |||
| Molar mass | 151.121 g·mol−1 | ||
| Appearance | slightly yellowish crystalline powder | ||
| Density | 1.546 g/cm3[1] | ||
| Melting point | 103 to 106 °C (217 to 223 °F; 376 to 379 K)[2] | ||
| Boiling point | 300 °C (572 °F; 573 K)[1] | ||
| -66.57·10−6 cm3/mol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Hazards | |||
| GHS pictograms |  [2] [2]
| ||
| H317, H319 | |||
| P280, P305+351+338 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
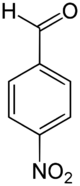
4-Nitrobenzaldehyde is an organic aromatic compound containing a nitro group para-substituted to an aldehyde.
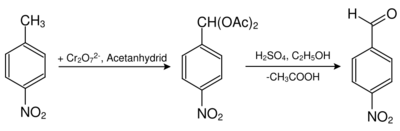
Nitrobenzyldiacetate is obtained by the reaction of 4-nitrotoluene and chromium(VI) oxide in acetic anhydride. 4-Nitrobenzaldehyde is obtained by hydrolysis by sulfuric acid in ethanol.[3]
References
- ↑ 1.0 1.1 4-Nitrobenzaldehyde(555-16-8) (Date Accessed 17 April 2013)
- ↑ 2.0 2.1 Template:Aldrich
- ↑ "p-NITROBENZALDEHYDE". Organic Syntheses 18: 61. 1938. doi:10.15227/orgsyn.018.0061. http://orgsyn.org/demo.aspx?prep=CV2P0441.
 |