Biology:SSI protease inhibitor
| SSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
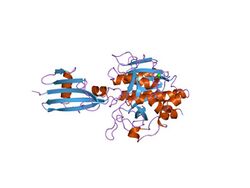 Molecular recognition at the active site of subtilisin BPN': crystallographic studies using genetically engineered protease inhibitor SSI (Streptomyces subtilisin inhibitor) | |||||||||
| Identifiers | |||||||||
| Symbol | SSI | ||||||||
| Pfam | PF00720 | ||||||||
| InterPro | IPR000691 | ||||||||
| PROSITE | PDOC00766 | ||||||||
| MEROPS | I16 | ||||||||
| SCOP2 | 2sic / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology the protein SSI is a Subtilisin inhibitor-like which stands for Streptomyces subtilisin inhibitor. This is a protease inhibitor. These are often synthesised as part of a larger precursor protein, either as a prepropeptide. The function of this protein domain is to prevent access of the substrate to the active site. It is found only in bacteria.
Function
SSI is a protease inhibitor, it prevents enzymes from acting on a substrate. Some SSI's also inhibit trypsin, chymotrypsin and griselysin.[1][2] Commercially, SSI's have huge potential in the commercial market, they help stabilise proteases in products such as laundry detergents to prevent autolysis of biological washing powders.[3] This means that the enzymes in the washing powder are kept in optimum performance.
Structure
SSI is a homodimer, in other words, it is made of two subunits which are exactly the same as each other. Each monomer contains 2 antiparallel beta-sheets and 2 short alpha-helices. Protease binding induces the widening of a channel-like structure, in which hydrophobic side-chains are sandwiched between 2 lobes.[4]
Studies have shown that the loss of the C-terminal domain reduces the inhibitory effect of the proteins. This implies that the C-terminal domain is responsible for maintaining the correct 3D fold.[5]
Structural similarities between the primary and secondary contact loops of SSI, and the ovomucoid and pancreatic secretory trypsin inhibitor family suggest evolution of the 2 families from a common ancestor.[4]
References
- ↑ Rawlings ND; Tolle DP; Barrett AJ (March 2004). "Evolutionary families of peptidase inhibitors". Biochem. J. 378 (Pt 3): 705–16. doi:10.1042/BJ20031825. PMID 14705960.
- ↑ Kojima S; Nishiyama Y; Kumagai I; Miura K (March 1991). "Inhibition of subtilisin BPN' by reaction site P1 mutants of Streptomyces subtilisin inhibitor". J. Biochem. 109 (3): 377–82. doi:10.1093/oxfordjournals.jbchem.a123389. PMID 1908859.
- ↑ "Stabilized variant of Streptomyces subtilisin inhibitor and its use in stabilizing subtilisin BPN'.". Protein Eng Des Sel 17 (4): 333–9. 2004. doi:10.1093/protein/gzh045. PMID 15187224.
- ↑ 4.0 4.1 Hirono S; Akagawa H; Mitsui Y; Iitaka Y (September 1984). "Crystal structure at 2.6 A resolution of the complex of subtilisin BPN' with streptomyces subtilisin inhibitor". J. Mol. Biol. 178 (2): 389–414. doi:10.1016/0022-2836(84)90150-5. PMID 6387152.
- ↑ Sakai M; Odani S; Ikenaka T (March 1980). "Importance of the carboxyl-terminal four amino acid residues in the inhibitory activity of Streptomyces subtilisin inhibitor (with a revision of its carboxyl-terminal sequence)". J. Biochem. 87 (3): 891–8. doi:10.1093/oxfordjournals.jbchem.a132819. PMID 6993452.
 |

