Biology:Protease inhibitor
In biology and biochemistry, protease inhibitors, or antiproteases,[1] are molecules that inhibit the function of proteases (enzymes that aid the breakdown of proteins). Many naturally occurring protease inhibitors are proteins.[2]
In medicine, protease inhibitor is often used interchangeably with alpha 1-antitrypsin (A1AT, which is abbreviated PI for this reason).[3] A1AT is indeed the protease inhibitor most often involved in disease, namely in alpha-1 antitrypsin deficiency.
Classification
Protease inhibitors may be classified either by the type of protease they inhibit, or by their mechanism of action. In 2004 Rawlings and colleagues introduced a classification of protease inhibitors based on similarities detectable at the level of amino acid sequence.[4] This classification initially identified 48 families of inhibitors that could be grouped into 26 related superfamily (or clans) by their structure. According to the MEROPS database there are now 81 families of inhibitors. These families are named with an I followed by a number, for example, I14 contains hirudin-like inhibitors.
By protease
Classes of proteases are:
- Aspartic protease inhibitors
- Cysteine protease inhibitors
- Metalloprotease inhibitors
- Serine protease inhibitors
- Threonine protease inhibitors
- Trypsin inhibitors
By mechanism
Classes of inhibitor mechanisms of action are:
- Suicide inhibitor
- Transition state inhibitor
- Protein protease inhibitor (see serpins)
- Chelating agents
Families
Inhibitor I4
This is a family of protease suicide inhibitors called the serpins. It contains inhibitors of multiple cysteine and serine protease families. Their mechanism of action relies on undergoing a large conformational change which inactivates their target's catalytic triad.
Inhibitor I9
| Peptidase inhibitor I9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
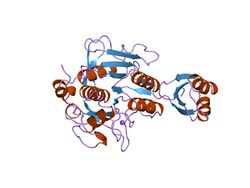 subtilisin bpn' prosegment (77 residues) complexed with a mutant subtilisin bpn' (266 residues). crystal ph 4.6. crystallization temperature 20 c diffraction temperature-160 c | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I9 | ||||||||
| Pfam | PF05922 | ||||||||
| InterPro | IPR010259 | ||||||||
| MEROPS | I9 | ||||||||
| SCOP2 | 1gns / SCOPe / SUPFAM | ||||||||
| |||||||||
Proteinase propeptide inhibitors (sometimes referred to as activation peptides) are responsible for the modulation of folding and activity of the peptidase pro-enzyme or zymogen. The pro-segment docks into the enzyme, shielding the substrate binding site, thereby promoting inhibition of the enzyme. Several such propeptides share a similar topology, despite often low sequence identities.[5][6] The propeptide region has an open-sandwich antiparallel-alpha/antiparallel-beta fold, with two alpha-helices and four beta-strands with a (beta/alpha/beta)x2 topology. The peptidase inhibitor I9 family contains the propeptide domain at the N-terminus of peptidases belonging to MEROPS family S8A, subtilisins. The propeptide is removed by proteolytic cleavage; removal activating the enzyme.
Inhibitor I10
| Serine endopeptidase inhibitors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 solution structure of marinostatin, a protease inhibitor, containing two ester linkages | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I10 | ||||||||
| Pfam | PF12559 | ||||||||
| InterPro | IPR022217 | ||||||||
| |||||||||
This family includes both microviridins and marinostatins. It seems likely that in both cases it is the C-terminus which becomes the active inhibitor after post-translational modifications of the full length, pre-peptide. It is the ester linkages within the key, 12-residue region that circularise the molecule giving it its inhibitory conformation.
Inhibitor I24
| PinA peptidase inhibitor | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Inhibitor_I24 | ||||||||
| Pfam | PF10465 | ||||||||
| InterPro | IPR019506 | ||||||||
| MEROPS | I24 | ||||||||
| |||||||||
This family includes PinA, which inhibits the endopeptidase La. It binds to the La homotetramer but does not interfere with the ATP binding site or the active site of La.
Inhibitor I29
| Cathepsin propeptide inhibitor domain (I29) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
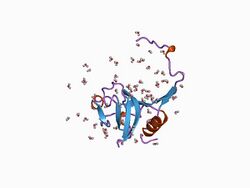 crystal structure of a cysteine protease proform | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I29 | ||||||||
| Pfam | PF08246 | ||||||||
| InterPro | IPR013201 | ||||||||
| |||||||||
The inhibitor I29 domain, which belongs to MEROPS peptidase inhibitor family I29, is found at the N-terminus of a variety of peptidase precursors that belong to MEROPS peptidase subfamily C1A; these include cathepsin L, papain, and procaricain.[7] It forms an alpha-helical domain that runs through the substrate-binding site, preventing access. Removal of this region by proteolytic cleavage results in activation of the enzyme. This domain is also found, in one or more copies, in a variety of cysteine peptidase inhibitors such as salarin.[8]
Inhibitor I34
| Saccharopepsin inhibitor I34 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
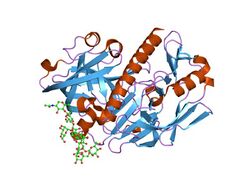 the structure of proteinase a complexed with an ia3 mutant inhibitor | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I34 | ||||||||
| Pfam | PF10466 | ||||||||
| InterPro | IPR019507 | ||||||||
| MEROPS | I34 | ||||||||
| |||||||||
The saccharopepsin inhibitor I34 is highly specific for the aspartic peptidase saccharopepsin. In the absence of saccharopepsin it is largely unstructured,[9] but in its presence, the inhibitor undergoes a conformational change forming an almost perfect alpha-helix from Asn2 to Met32 in the active site cleft of the peptidase.
Inhibitor I36
| Peptidase inhibitor family I36 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Error creating thumbnail: Unable to save thumbnail to destination the 3d structure of the streptomyces metalloproteinase inhibitor, smpi, isolated from streptomyces nigrescens tk-23, nmr, minimized average structure | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I36 | ||||||||
| Pfam | PF03995 | ||||||||
| Pfam clan | CL0333 | ||||||||
| InterPro | IPR007141 | ||||||||
| MEROPS | I36 | ||||||||
| SCOP2 | 1bhu / SCOPe / SUPFAM | ||||||||
| |||||||||
The peptidase inhibitor family I36 domain is only found in a small number of proteins restricted to Streptomyces species. All have four conserved cysteines that probably form two disulphide bonds. One of these proteins from Streptomyces nigrescens, is the well characterised metalloproteinase inhibitor SMPI.[10][11]
The structure of SMPI has been determined. It has 102 amino acid residues with two disulphide bridges and specifically inhibits metalloproteinases such as thermolysin, which belongs to MEROPS peptidase family M4. SMPI is composed of two beta-sheets, each consisting of four antiparallel beta-strands. The structure can be considered as two Greek key motifs with 2-fold internal symmetry, a Greek key beta-barrel. One unique structural feature found in SMPI is in its extension between the first and second strands of the second Greek key motif which is known to be involved in the inhibitory activity of SMPI. In the absence of sequence similarity, the SMPI structure shows clear similarity to both domains of the eye lens crystallins, both domains of the calcium sensor protein-S, as well as the single-domain yeast killer toxin. The yeast killer toxin structure was thought to be a precursor of the two-domain beta gamma-crystallin proteins, because of its structural similarity to each domain of the beta gamma-crystallins. SMPI thus provides another example of a single-domain protein structure that corresponds to the ancestral fold from which the two-domain proteins in the beta gamma-crystallin superfamily are believed to have evolved.[12]
Inhibitor I42
| Chagasin family peptidase inhibitor I42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
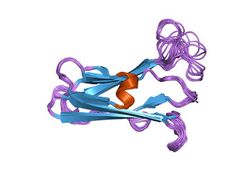 solution structure of the trypanosoma cruzi cysteine protease inhibitor chagasin | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I42 | ||||||||
| Pfam | PF09394 | ||||||||
| InterPro | IPR018990 | ||||||||
| MEROPS | I42 | ||||||||
| |||||||||
Inhibitor family I42 includes chagasin, a reversible inhibitor of papain-like cysteine proteases.[13] Chagasin has a beta-barrel structure, which is a unique variant of the immunoglobulin fold with homology to human CD8alpha.[14][15]
Inhibitor I48
| Peptidase inhibitor clitocypin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Inhibitor_I48 | ||||||||
| Pfam | PF10467 | ||||||||
| InterPro | IPR019508 | ||||||||
| MEROPS | I48 | ||||||||
| |||||||||
Inhibitor family I48 includes clitocypin, which binds and inhibits cysteine proteinases. It has no similarity to any other known cysteine proteinase inhibitors but bears some similarity to a lectin-like family of proteins from mushrooms.[16]
Inhibitor I53
| Thrombin inhibitor Madanin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Inhibitor_I53 | ||||||||
| Pfam | PF11714 | ||||||||
| InterPro | IPR021716 | ||||||||
| MEROPS | I53 | ||||||||
| |||||||||
Members of this family are the peptidase inhibitor madanin proteins. These proteins were isolated from tick saliva.[17]
Inhibitor I67
| Bromelain inhibitor VI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 nmr structure of bromelain inhibitor vi from pineapple stem | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I67 | ||||||||
| Pfam | PF11405 | ||||||||
| InterPro | IPR022713 | ||||||||
| MEROPS | I67 | ||||||||
| |||||||||
Bromelain inhibitor VI, in the Inhibitor I67 family, is a double-chain inhibitor consisting of an 11-residue and a 41-residue chain.
Inhibitor I68
| Carboxypeptidase inhibitor I68 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Error creating thumbnail: Unable to save thumbnail to destination crystal structure of the tick carboxypeptidase inhibitor in complex with human carboxypeptidase b | |||||||||
| Identifiers | |||||||||
| Symbol | Inhibitor_I68 | ||||||||
| Pfam | PF10468 | ||||||||
| InterPro | IPR019509 | ||||||||
| MEROPS | I68 | ||||||||
| |||||||||
The Carboxypeptidase inhibitor I68 family represents a family of tick carboxypetidase inhibitors.
Inhibitor I78
| Peptidase inhibitor I78 family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Inhibitor_I78 | ||||||||
| Pfam | PF11720 | ||||||||
| Pfam clan | CL0367 | ||||||||
| InterPro | IPR021719 | ||||||||
| MEROPS | I78 | ||||||||
| |||||||||
The peptidase inhibitor I78 family includes Aspergillus elastase inhibitor.
Compounds
- Aprotinin
- Bestatin
- Calpain inhibitor I and II
- Chymostatin
- E-64
- Leupeptin (N-acetyl-L-leucyl-L-leucyl-L-argininal)
- alpha-2-Macroglobulin
- Pefabloc SC
- Pepstatin
- PMSF (phenylmethanesulfonyl fluoride)
- TLCK
- Trypsin inhibitors
See also
References
- ↑ "antiprotease". https://medical-dictionary.thefreedictionary.com/antiprotease.
- ↑ Roy, Mrinalini; Rawat, Aadish; Kaushik, Sanket; Jyoti, Anupam; Srivastava, Vijay Kumar (2022-08-01). "Endogenous cysteine protease inhibitors in upmost pathogenic parasitic protozoa" (in en). Microbiological Research 261: 127061. doi:10.1016/j.micres.2022.127061. ISSN 0944-5013. PMID 35605309.
- ↑ OMIM - PROTEASE INHIBITOR 1; PI
- ↑ "Evolutionary families of peptidase inhibitors". Biochem. J. 378 (Pt 3): 705–16. March 2004. doi:10.1042/BJ20031825. PMID 14705960.
- ↑ "Solution structure of the pro-hormone convertase 1 pro-domain from Mus musculus". J. Mol. Biol. 320 (4): 801–12. July 2002. doi:10.1016/S0022-2836(02)00543-0. PMID 12095256.
- ↑ "The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0 A resolution". J. Mol. Biol. 284 (1): 137–44. November 1998. doi:10.1006/jmbi.1998.2161. PMID 9811547.
- ↑ "The prosequence of procaricain forms an alpha-helical domain that prevents access to the substrate-binding cleft". Structure 4 (10): 1193–203. October 1996. doi:10.1016/s0969-2126(96)00127-x. PMID 8939744.
- ↑ "A new type of cysteine proteinase inhibitor--the salarin gene from Atlantic salmon (Salmo salar L.) and Arctic charr (Salvelinus alpinus)". Biochimie 85 (7): 677–81. July 2003. doi:10.1016/S0300-9084(03)00128-7. PMID 14505823.
- ↑ "IA3, an aspartic proteinase inhibitor from Saccharomyces cerevisiae, is intrinsically unstructured in solution". Biochemistry 43 (14): 4071–81. April 2004. doi:10.1021/bi034823n. PMID 15065849.
- ↑ "Nucleotide sequence of the gene for a metalloproteinase inhibitor of Streptomyces nigrescens (SMPI)". Nucleic Acids Res. 18 (21): 6433. November 1990. doi:10.1093/nar/18.21.6433. PMID 2243793.
- ↑ "Amino acid sequence of Streptomyces metallo-proteinase inhibitor from Streptomyces nigrescens TK-23". J. Biochem. 97 (1): 173–80. January 1985. doi:10.1093/oxfordjournals.jbchem.a135041. PMID 3888972.
- ↑ "NMR structure of the Streptomyces metalloproteinase inhibitor, SMPI, isolated from Streptomyces nigrescens TK-23: another example of an ancestral beta gamma-crystallin precursor structure". J. Mol. Biol. 282 (2): 421–33. September 1998. doi:10.1006/jmbi.1998.2022. PMID 9735297.
- ↑ "Identification, characterization and localization of chagasin, a tight-binding cysteine protease inhibitor in Trypanosoma cruzi". J. Cell Sci. 114 (Pt 21): 3933–42. November 2001. doi:10.1242/jcs.114.21.3933. PMID 11719560.
- ↑ Figueiredo da Silva AA; de Carvalho Vieira L; Krieger MA; Goldenberg S; Zanchin NI; Guimarães BG (February 2007). "Crystal structure of chagasin, the endogenous cysteine-protease inhibitor from Trypanosoma cruzi". J. Struct. Biol. 157 (2): 416–23. doi:10.1016/j.jsb.2006.07.017. PMID 17011790.
- ↑ "The structure of chagasin in complex with a cysteine protease clarifies the binding mode and evolution of an inhibitor family". Structure 15 (5): 535–43. May 2007. doi:10.1016/j.str.2007.03.012. PMID 17502099.
- ↑ "Clitocypin, a new type of cysteine proteinase inhibitor from fruit bodies of mushroom clitocybe nebularis". J. Biol. Chem. 275 (26): 20104–9. June 2000. doi:10.1074/jbc.M001392200. PMID 10748021.
- ↑ "Identification and characterization of novel salivary thrombin inhibitors from the ixodidae tick, Haemaphysalis longicornis". Eur. J. Biochem. 270 (9): 1926–34. May 2003. doi:10.1046/j.1432-1033.2003.03560.x. PMID 12709051.
External links
